Food Scientist's Guide to Fats and Oils for Margarine And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tailored Microstructure of Colloidal Lipid Particles for Pickering Emulsions with Tunable Properties
Electronic Supplementary Material (ESI) for Soft Matter. This journal is © The Royal Society of Chemistry 2017 Supporting information. Tailored Microstructure of Colloidal Lipid Particles for Pickering Emulsions with Tunable Properties Anja A.J. Schröder1,2, Joris Sprakel2, Karin Schroën1, Claire C. Berton-Carabin1 1. Laboratory of Food Process Engineering, Wageningen University and Research, Bornse Weilanden 9, 6708 WG Wageningen, The Netherlands. 2. Physical Chemistry and Soft Matter, Wageningen University and Research, Stippeneng 4, 6708 WE Wageningen, The Netherlands. Email addresses: [email protected], [email protected], [email protected], [email protected] S1 S1. Tripalmitin CLP dispersions produced with 0.5% w/w (left) and 1% w/w (right) Tween40 in the aqueous phase. S2. Fatty acid composition of palm stearin. Component name Percentage average C14:0 1.16 C16:0 82.18 C18:0 5.12 C18:1 9.03 C18:2 1.83 C18:3 0.02 C20:0 0.32 C22:0 0.25 C22:1 0.04 C24:0 0.05 S3. Hydrodynamic diameter (z-average) measured by dynamic light scattering, and, D[4,3], D[3,2], D10, D50 and D90 measured by static light scattering of CLPs composed of (a) tripalmitin, (b) tripalmitin/tricaprylin 4:1, (c) palm stearin. S2 Type of z-average PdI D[4,3] A D[3,2] D10 D50 D90 lipid (μm) (μm) (μm) (μm) (μm) (μm) Tripalmitin 0.162 ± 0.112 ± 0.130 ± 0.118 ± 0.082 ± 0.124 ± 0.184 ± 0.005 0.02 0.004 0.003 0.002 0.004 0.005 Tripalmitin/ 0.130 ± 0.148 ± 0.131 ± 0.117 ± 0.080 ± 0.124 ± 0.191 ± tricaprylin 0.011 0.02 0.003 0.001 0.004 0.002 0.010 (4:1) Palm 0.158 ± 0.199 ± 0.133 ± 0.119 ± 0.081 ± 0.127 ± 0.195 ± stearin 0.007 0.02 0.002 0.002 0.005 0.000 0.010 A B S4. -

Essential Wholesale & Labs Carrier Oils Chart
Essential Wholesale & Labs Carrier Oils Chart This chart is based off of the virgin, unrefined versions of each carrier where applicable, depending on our website catalog. The information provided may vary depending on the carrier's source and processing and is meant for educational purposes only. Viscosity Absorbtion Comparible Subsitutions Carrier Oil/Butter Color (at room Odor Details/Attributes Rate (Based on Viscosity & Absorbotion Rate) temperature) Description: Stable vegetable butter with a neutral odor. High content of monounsaturated oleic acid and relatively high content of natural antioxidants. Offers good oxidative stability, excellent Almond Butter White to pale yellow Soft Solid Fat Neutral Odor Average cold weather stability, contains occlusive properties, and can act as a moistening agent. Aloe Butter, Illipe Butter Fatty Acid Compositon: Palmitic, Stearic, Oleic, and Linoleic Description: Made from Aloe Vera and Coconut Oil. Can be used as an emollient and contains antioxidant properties. It's high fluidiy gives it good spreadability, and it can quickly hydrate while Aloe Butter White Soft Semi-Solid Fat Neutral Odor Average being both cooling and soothing. Fatty Acid Almond Butter, Illipe Butter Compostion: Linoleic, Oleic, Palmitic, Stearic Description: Made from by combinging Aloe Vera Powder with quality soybean oil to create a Apricot Kernel Oil, Broccoli Seed Oil, Camellia Seed Oil, Evening Aloe Vera Oil Clear, off-white to yellow Free Flowing Liquid Oil Mild musky odor Fast soothing and nourishing carrier oil. Fatty Acid Primrose Oil, Grapeseed Oil, Meadowfoam Seed Oil, Safflower Compostion: Linoleic, Oleic, Palmitic, Stearic Oil, Strawberry Seed Oil Description: This oil is similar in weight to human sebum, making it extremely nouirshing to the skin. -
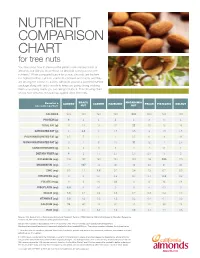
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Information About Oils the National Edible Oil Distributors’ Association
Information About Oils The National Edible Oil Distributors’ Association Sunflower Oil Sunflower Oil - Information About Oils Introduction Nutritional Profile Whilst the vibrant strong sunflower is recognised Due to its nutritional profile, sunflower oil can bear worldwide for its beauty, it is also an important the following nutrition claims: source of food. Sunflower oil is a valued and Nutrition Claims High polyunsaturated fat (more healthy vegetable oil and sunflower seeds are en- than 45% of the fatty acids are from polyunsaturat- joyed as a healthy, tasty snack and nutritious ingre- ed fats, which represent more than 20% of the en- dient to many foods. ergy content) High unsaturated fat (more than 70% Sunflower oil is obtained from the seeds within the of the fatty acids are from unsaturated fats, which brown hub in the centre of the sunflower plant. represent more than 20% of the energy content) Each flower can develop up to 2000 sunflower High vitamin E (more than 30% of Recommended seeds. The oil is pale yellow in colour but contains Daily Allowances (RDA) of vitamin E set at 12 mg/day a level of natural waxes that give the oil a ‘cloudy’ Health claims – Positive EFSA opinion Linoleic acid appearance at cooler temperatures. These waxes (omega-6 fatty acids) contributes to the mainte- can be removed by a process commonly known as nance of normal blood cholesterol concentrations. winterisation. Essential fatty acids (omega-3 and omega-6 fatty The wild sunflower is native to North America but acids) are needed for the normal growth of children. commercialisation of the plant took place in Rus- Vitamin E protects lipids, proteins and DNA against sia. -

Chinese Express Ingredients and Allergen Information
Asian Ingredient and Allergen Information Item Name Ingredient Statement Allergen Statement Appetizers Chicken Egg Roll (Cabbage, Enriched Bleached Flour [{Wheat Flour, Niacin, Reduced Iron, Thiamine Mononitrate, Riboflavin, Folic Acid}, Calcium Propionate, Malted Barley Flour], Water, White Meat Chicken, Carrots, Onion, Textured Soy Flour, Celery, Enriched Durum Flour [Wheat Flour, Niacin, Ferrous Sulfate, Thiamine Mononitrate, Riboflavin, Folic Acid], Contains 2% or less of: Vegetable Oil [Soybean, Cottonseed, Corn, and/or Canola Oil], Salt, Autolyzed Yeast Extract, Modified Food Starch, Sugar, Garlic, Dried Whole Egg, Soy Sauce Powder [Soy Sauce {Wheat, Soybeans, Salt}, Maltodextrin, Salt], Wheat Gluten, Spice, Cornstarch. Fried in Vegetable Oil [Soybean, Cottonseed, Corn, and/or Canola Oil]), Soybean Oil (Hydrogenated Soybean Oil Chicken Egg Roll with TBHQ and Citric Acid added as preservatives, and Dimethylpolysiloxane added as an anti-foaming agent). Contains Egg, Soy, Wheat. Vegetable Egg Roll (Cabbage, Enriched Flour [{Bleached Wheat Flour, Niacin, Reduced Iron, Thiamine Mononitrate, Riboflavin, Folic Acid}, Calcium Propionate, Malted Barley Flour], Water, Carrots, Broccoli, Water Chestnuts, Enriched Durum Flour [Wheat Flour, Niacin, Ferrous Sulfate, Thiamine Mononitrate, Riboflavin, Folic Acid], Brown Rice, Celery, Bamboo Shoots, Contains 2% or Less of: Salt, Modified Food Starch, Onion, Yeast Extract [Yeast Extract, Sunflower Oil], Toasted Sesame Oil, Flavoring [Yeast Extract, Salt, Maltodextrin, Natural and Artificial Flavor], Vegetable Oil [Cottonseed and/or Canola Oil], Dried Garlic, Wheat Gluten, Sugar, Spice, Eggs, Cornstarch. Fried in Vegetable Oil (Cottonseed and/or Canola Oil), Soybean Oil (Hydrogenated Veggie Egg Roll Soybean Oil with TBHQ and Citric Acid added as preservatives, and Dimethylpolysiloxane added as an anti-foaming agent). Contains Egg, Wheat. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0022685 A1 Buffy Et Al
US 2010.0022685A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0022685 A1 Buffy et al. (43) Pub. Date: Jan. 28, 2010 (54) RAPID DRY FIBERGLASS STAIN Related U.S. Application Data (75) Inventors: Jarrod J. Buffy, Waterville, OH (60) Provisional application No. 61/083,002, filed on Jul. (US); William V. Pagryzinski, Leo, 23, 2008. IN (US) Publication Classification Correspondence Address: (51) Int. Cl. CALFEE HALTER & GRISWOLD, LLP C09D 5/00 (2006.01) 800 SUPERIORAVENUE, SUITE 1400 (52) U.S. Cl. ........................................................ 523/400 CLEVELAND, OH 44114 (US) (57) ABSTRACT (73) Assignee: THERMA-TRU CORP., Maumee, A portion of the linseed oil ingredient used in fiberglass OH (US) composite stains is replaced with a modified Cashew Nut Shell Liquid (CNSL) resin. As a result, the drying time of the (21) Appl. No.: 12/505,702 stain is shortened considerably yet is still long enough to allow working of the stain into the composite for developing (22) Filed: Jul. 20, 2009 a simulated wood appearance. US 2010/0022685 A1 Jan. 28, 2010 RAPID DRY FIBERGLASS STAN 0007 Accordingly, this invention provides a new stain for fiberglass composite Substrates comprising a colorant and a resin-forming component, the resin-forming component 0001. This application claims the benefit of U.S. Provi comprising a linseed oil ingredient and a modified CNSL sional patent application Ser. No. 61/083,002 filed on Jul. 23, CS1. 2008, for RAPID DRY FIBERGLASS STAIN, the entire disclosure of which is fully incorporated herein by reference. DETAILED DESCRIPTION Fiberglass Composite BACKGROUND 0008. This invention is directed to making stains for fiber 0002. -

Zahnmedizinische Produkte Und Ihre Inhaltsstoffe
Medikamente Zahnmedizinische Produkte III–8.3 Zahnmedizinische Produkte und ihre Inhaltsstoffe Produktname Anbieter/ Inhaltsstoffe Verwendung Hersteller/ (Elemente in Massen-%, Vertrieb seit x = keine Angabe) Adaptic LC Johnson & John Ba-Glas Kunststoff son AH 26 DeTrey Dentsply Pulver: Silber, Wismutoxid, Methenamin, Wurzelkanal- Titandioxid, Harz: Epoxybisphenolharz Füllungsmasse Alba KF Heraeus/Heraeus/ Pd: 40,0; Ag: 53,0; Sn: x; Zn: 3,5; In: 2,0 Zahnlegierung 1988 Alba Lot 810 Heraeus Kulzer Au: 10,00; Pd: 1,00; Cu: 11,00; Ag: 69,00; Dentallot In: 6,00; Zn: 3,00 Alba Lot 880 Heraeus Kulzer Au: 12,00; Pd: 4,00; Cu: 11,00; Ag: 69,00; Dentallot In: 4,00 Alba SG Heraeus/Heraeus/ Au: 10,0; Pd: 21,0; Ag: 57,0; Cu: 10,0; Zn: Zahnlegierung 1953 2,0 Albabond Heraeus/Heraeus/ Pd: 60,5; Ag: 28,0; Sn: 3,0; In: 7,0; Ga: x Zahnlegierung 1980 Albabond A Heraeus/Heraeus/ Pd: 57,0; Ag: 32,8; Sn: 6,8; In: 3,4 Zahnlegierung 1989 Albabond E Heraeus/Heraeus/ Au: 1,6; Pd: 78,4; Cu: 11,0; Sn: x; In: x; Ga: Zahnlegierung 1983 7,5 Albabond EH Heraeus/Heraeus/ Au: 2,0; Pd: 79,0; Cu: 10,0; Ga: 8,8 Zahnlegierung 1984 Albabond GF Heraeus/Heraeus/ Pd: 60,5; Ag: 19,5; Cu: 7,5; Sn: 3,0; In: 9,5 Zahnlegierung 1987 Albabond U Heraeus/Heraeus/ Au: 2,0; Pd: 75,0; Cu: 9,5; Sn: 3,0; In: 7,0; Zahnlegierung 1982 Ga: 3,5 Albiotic Upjohn Lincomycin Antibiotikum Aleram 2 Allgemeine Gold- Au: x; Pd: 78,5; Cu: 10,5; Sn: x; Ga: 8,0 Zahnlegierung u. -

Sauces Reconsidered
SAUCES RECONSIDERED Rowman & Littlefield Studies in Food and Gastronomy General Editor: Ken Albala, Professor of History, University of the Pacific ([email protected]) Rowman & Littlefield Executive Editor: Suzanne Staszak-Silva ([email protected]) Food studies is a vibrant and thriving field encompassing not only cooking and eating habits but also issues such as health, sustainability, food safety, and animal rights. Scholars in disciplines as diverse as history, anthropol- ogy, sociology, literature, and the arts focus on food. The mission of Row- man & Littlefield Studies in Food and Gastronomy is to publish the best in food scholarship, harnessing the energy, ideas, and creativity of a wide array of food writers today. This broad line of food-related titles will range from food history, interdisciplinary food studies monographs, general inter- est series, and popular trade titles to textbooks for students and budding chefs, scholarly cookbooks, and reference works. Appetites and Aspirations in Vietnam: Food and Drink in the Long Nine- teenth Century, by Erica J. Peters Three World Cuisines: Italian, Mexican, Chinese, by Ken Albala Food and Social Media: You Are What You Tweet, by Signe Rousseau Food and the Novel in Nineteenth-Century America, by Mark McWilliams Man Bites Dog: Hot Dog Culture in America, by Bruce Kraig and Patty Carroll A Year in Food and Beer: Recipes and Beer Pairings for Every Season, by Emily Baime and Darin Michaels Celebraciones Mexicanas: History, Traditions, and Recipes, by Andrea Law- son Gray and Adriana Almazán Lahl The Food Section: Newspaper Women and the Culinary Community, by Kimberly Wilmot Voss Small Batch: Pickles, Cheese, Chocolate, Spirits, and the Return of Artisanal Foods, by Suzanne Cope Food History Almanac: Over 1,300 Years of World Culinary History, Cul- ture, and Social Influence, by Janet Clarkson Cooking and Eating in Renaissance Italy: From Kitchen to Table, by Kath- erine A. -

Olive Oil Jars Left Behind By
live oil jars left behind by the ancient Greeks are testament to our centuries- old use of cooking oil. Along with salt and pepper, oil Oremains one of the most important and versatile tools in your kitchen. It keeps food from sticking to pans, adds flavor and moisture, and conducts the heat that turns a humble stick of potato into a glorious french fry. Like butter and other fats, cooking oil also acts as a powerful solvent, unleashing fat-soluble nutrients and flavor compounds in everything from tomatoes and onions to spices and herbs. It’s why so many strike recipes begin with heating garlic in oil rather than, say, simmering it in water. The ancient Greeks didn’t tap many cooking oils. (Let’s see: olive oil, olive oil, or—ooh, this is exciting!—how about olive oil?) But you certainly can. From canola to safflower to grapeseed to walnut, each oil has its own unique flavor (or lack thereof), aroma, and optimal cooking temperature. Choosing the right kind for the task at hand can save you money, boost your health, and improve your cooking. OK, so you probably don’t stop to consider your cooking oil very often. But there’s a surprising amount to learn about What’s this? this liquid gold. BY VIRGINIAWILLIS Pumpkin seed oil suspended in corn oil—it looks like a homemade Lava Lamp! 84 allrecipes.com PHOTOS BY KATE SEARS WHERE TO store CANOLA OIL GRAPESEED OIL are more likely to exhibit the characteristic YOUR OIL flavor and aroma of their base nut or seed. -

Some of the Factors Influencing the Growth of Molds in Butter Harold Macy Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1929 Some of the factors influencing the growth of molds in butter Harold Macy Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agriculture Commons, and the Food Science Commons Recommended Citation Macy, Harold, "Some of the factors influencing the growth of molds in butter " (1929). Retrospective Theses and Dissertations. 14244. https://lib.dr.iastate.edu/rtd/14244 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the miaofilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. in the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Al.so, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are reproduced by sectioning the original, beginning at the upper left-hand comer and continuing from left to right in equal sections vwth small overiaps. -
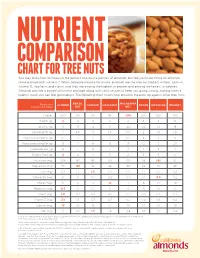
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Soy Free Diet Avoiding Soy
SOY FREE DIET AVOIDING SOY An allergy to soy is common in babies and young children, studies show that often children outgrow a soy allergy by age 3 years and the majority by age 10. Soybeans are a member of the legume family; examples of other legumes include beans, peas, lentils and peanut. It is important to remember that children with a soy allergy are not necessarily allergic to other legumes, request more clarification from your allergist if you are concerned. Children with a soy allergy may have nausea, vomiting, abdominal pain, diarrhea, bloody stool, difficulty breathing, and or a skin reaction after eating or drinking soy products. These symptoms can be avoided by following a soy free diet. What foods are not allowed on a soy free diet? Soy beans and edamame Soy products, including tofu, miso, natto, soy sauce (including sho yu, tamari), soy milk/creamer/ice cream/yogurt, soy nuts and soy protein, tempeh, textured vegetable protein (TVP) Caution with processed foods - soy is widely used manufactured food products – remember to carefully read labels. o Soy products and derivatives can be found in many foods, including baked goods, canned tuna and meat, cereals, cookies, crackers, high-protein energy bars, drinks and snacks, infant formulas, low- fat peanut butter, processed meats, sauces, chips, canned broths and soups, condiments and salad dressings (Bragg’s Liquid Aminos) USE EXTRA CAUTION WITH ASIAN CUISINE: Asian cuisine are considered high-risk for people with soy allergy due to the common use of soy as an ingredient and the possibility of cross-contamination, even if a soy-free item is ordered.