Sclerocona Acutella (Eversmann) (Crambidae: Pyraustinae), Naturalized Along the Easte Rn Seaboard
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fauna Lepidopterologica Volgo-Uralensis" 150 Years Later: Changes and Additions
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2000) 31 (1/2):327-367< Würzburg, ISSN 0171-0079 "Fauna lepidopterologica Volgo-Uralensis" 150 years later: changes and additions. Part 5. Noctuidae (Insecto, Lepidoptera) by Vasily V. A n ik in , Sergey A. Sachkov , Va d im V. Z o lo t u h in & A n drey V. Sv ir id o v received 24.II.2000 Summary: 630 species of the Noctuidae are listed for the modern Volgo-Ural fauna. 2 species [Mesapamea hedeni Graeser and Amphidrina amurensis Staudinger ) are noted from Europe for the first time and one more— Nycteola siculana Fuchs —from Russia. 3 species ( Catocala optata Godart , Helicoverpa obsoleta Fabricius , Pseudohadena minuta Pungeler ) are deleted from the list. Supposedly they were either erroneously determinated or incorrect noted from the region under consideration since Eversmann 's work. 289 species are recorded from the re gion in addition to Eversmann 's list. This paper is the fifth in a series of publications1 dealing with the composition of the pres ent-day fauna of noctuid-moths in the Middle Volga and the south-western Cisurals. This re gion comprises the administrative divisions of the Astrakhan, Volgograd, Saratov, Samara, Uljanovsk, Orenburg, Uralsk and Atyraus (= Gurjev) Districts, together with Tataria and Bash kiria. As was accepted in the first part of this series, only material reliably labelled, and cover ing the last 20 years was used for this study. The main collections are those of the authors: V. A n i k i n (Saratov and Volgograd Districts), S. -

Harmful Non-Indigenous Species in the United States
Harmful Non-Indigenous Species in the United States September 1993 OTA-F-565 NTIS order #PB94-107679 GPO stock #052-003-01347-9 Recommended Citation: U.S. Congress, Office of Technology Assessment, Harmful Non-Indigenous Species in the United States, OTA-F-565 (Washington, DC: U.S. Government Printing Office, September 1993). For Sale by the U.S. Government Printing Office ii Superintendent of Documents, Mail Stop, SSOP. Washington, DC 20402-9328 ISBN O-1 6-042075-X Foreword on-indigenous species (NIS)-----those species found beyond their natural ranges—are part and parcel of the U.S. landscape. Many are highly beneficial. Almost all U.S. crops and domesticated animals, many sport fish and aquiculture species, numerous horticultural plants, and most biologicalN control organisms have origins outside the country. A large number of NIS, however, cause significant economic, environmental, and health damage. These harmful species are the focus of this study. The total number of harmful NIS and their cumulative impacts are creating a growing burden for the country. We cannot completely stop the tide of new harmful introductions. Perfect screening, detection, and control are technically impossible and will remain so for the foreseeable future. Nevertheless, the Federal and State policies designed to protect us from the worst species are not safeguarding our national interests in important areas. These conclusions have a number of policy implications. First, the Nation has no real national policy on harmful introductions; the current system is piecemeal, lacking adequate rigor and comprehensiveness. Second, many Federal and State statutes, regulations, and programs are not keeping pace with new and spreading non-indigenous pests. -

DNA Barcoding and Morphology Reveal Three Cryptic Species of Anania
Systematic Entomology (2012), 37, 686–705 DNA barcoding and morphology reveal three cryptic species of Anania (Lepidoptera: Crambidae: Pyraustinae) in North America, all distinct from their European counterpart ZHAOFU YANG1,9, JEAN-FRANC¸ OIS LANDRY2,LOUIS HANDFIELD3, YALIN ZHANG1,M.ALMASOLIS4, DANIEL HANDFIELD5, BRIAN G. SCHOLTENS6, MARKO MUTANEN7, MATTHIAS NUSS8 and PAUL D. N. HEBERT9 1Key laboratory of Plant Protection Resources and Pest Management, Ministry of Education; Entomological Museum, Northwest A&F University, Yangling, China, 2Agriculture and Agri-Food Canada, Eastern Cereal and Oilseed Research Centre, C.E.F., Ottawa, Ontario K1A 0C6, Canada, 3133 rue Messier, #301, Mont-Saint-Hilaire, Quebec´ J3H 2W8, Canada, 4Systematic Entomology Laboratory, USDA, c/o Smithsonian Institution, National Museum Natural History, Washington, DC 20013-7012, U.S.A., 5Chemin des Grands Coteaux, Saint-Mathieu-de-Beloeil, Quebec,´ Canada, 6Department of Biology, College of Charleston, SC, U.S.A., 7Department of Biology, University of Oulu, Zoological Museum, Oulu, Finland, 8Museum of Zoology, Senckenberg Natural History Collections Dresden, Konigsbr¨ ucker¨ Landstrasse 159, 01109 Dresden, Germany and 9Biodiversity Institute of Ontario, University of Guelph, Guelph, Ontario N1G 2W1, Canada Abstract. Anania coronata (Hufnagel), a Holarctic species of pyraustine crambid moth, has long been treated as having two geographically separated subspecies – the nominotypical Anania coronata in the Palaearctic Region and Anania coronata tertialis (Guenee)´ in the Nearctic Region. Maximum likelihood and Bayesian inference analysis of mitochondrial DNA barcodes both recover four well-supported, reciprocally monophyletic groups within Anania coronata. Qualitative and quantitative analyses of genital structures reveal diagnostic differences that correspond to the four barcode lineages. On the basis of both molecular and morphological evidence, we conclude that Anania coronata is actually a complex of four species. -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 .................................. -

Wallkill River National Wildlife Refuge Comprehensive Conservation Plan February 2009 This Blue Goose, Designed by J.N
U.S. Fish & Wildlife Service Wallkill River National Wildlife Refuge Comprehensive Conservation Plan February 2009 This blue goose, designed by J.N. “Ding” Darling, has become the symbol of the National Wildlife Refuge System. The U.S. Fish and Wildlife Service is the principal federal agency responsible for conserving, protecting, and enhancing fi sh, wildlife, plants, and their habitats for the continuing benefi t of the American people. The Service manages the 97-million acre National Wildlife Refuge System comprised of more than 548 national wildlife refuges and thousands of waterfowl production areas. It also operates 69 national fi sh hatcheries and 81 ecological services fi eld stations. The agency enforces federal wildlife laws, manages migratory bird populations, restores nationally signifi cant fi sheries, conserves and restores wildlife habitat such as wetlands, administers the Endangered Species Act, and helps foreign governments with their conservation efforts. It also oversees the Federal Assistance Program which distributes hundreds of millions of dollars in excise taxes on fi shing and hunting equipment to state wildlife agencies. Comprehensive Conservation Plans provide long term guidance for management decisions and set forth goals, objectives, and strategies needed to accomplish refuge purposes and identify the Service’s best estimate of future needs. These plans detail program planning levels that are sometimes substantially above current budget allocations and, as such, are primarily for Service strategic planning and program prioritization purposes. The plans do not constitute a commitment for staffi ng increases, operational and maintenance increases, or funding for future land acquisition. U.S. Fish & Wildlife Service Wallkill River National Wildlife Refuge Comprehensive Conservation Plan February 2009 Submitted by: Edward Henry Date Refuge Manager Wallkill River National Wildlife Refuge Concurrence by: Janet M. -

Nota Lepidopterologica
Nota lepid. 28 (1): 17-23 17 ©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Revision of Evergestis anartalis (Staudinger, 1892) comb. rev. from Central Asia (Pyraloidea: Crambidae: Evergestinae) Matthias Nuss Museum für Tierkunde, Königsbrücker Landstr. 159, D-01109 Dresden, e-mail: [email protected] Abstract. The nomenclature of Evergestis anartalis (Staudinger, 1892) comb, rev., a species which has been described three times and of which the names have been placed in three different subfamilies of Crambidae, is investigated. The generic name Maelinoptera Staudinger, 1893 syn. n. is revised as a junior subjective synonym of Evergestis Hübner, 1825 and the type-species of this monotypic genus, Hercyna anartalis Staudinger, 1892, is transferred to Evergestis. Evergestis heliacalis Zerny, 1914 syn. n. and Noctuelia anartalis Hampson, 1918 syn. n., are considered as junior subjective synonyms of Evergestis anartalis (Staudinger, 1892) (Hercyna). Evergestis anartalis Hampson, 1918 (Noctuelia) therefore becomes a junior secondary homonym of Evergestis anartalis (Staudinger, 1892) (Hercyna). Lectotypes of Hercyna anartalis Staudinger, 1892 and Evergestis heliacalis Zerny, 1914 are designated. Evergestis anartalis (Staudinger, 1892) is redescribed; adults, male and female genitalia are illustrated. According to the specimens investigated, it is assumed that Evergestis anartalis is endemic to sub-alpine and alpine altitudes in Central Asia. Key words. Evergestis anartalis, taxonomic revision. Central Asia, alpine altitudes, endemic species. Introduction After Staudinger (1892: pi. 3 fig. 17) already figured Hercyna anartalis, he provided the description of this species a year later and described the genus Maelinoptera to include Hercyna anartalis only (Staudinger 1893: 72-73). -

Xyleninae 73.087 2385 Small Mottled Willow
Xyleninae 73.087 2385 Small Mottled Willow (Spodoptera exigua) 73.089 2386 Mediterranean Brocade (Spodoptera littoralis) 73.091 2396 Rosy Marbled (Elaphria venustula) 73.092 2387 Mottled Rustic (Caradrina morpheus) 73.093 2387a Clancy's Rustic (Caradrina kadenii) 73.095 2389 Pale Mottled Willow (Caradrina clavipalpis) 73.096 2381 Uncertain (Hoplodrina octogenaria) 73.0961 2381x Uncertain/Rustic agg. (Hoplodrina octogenaria/blanda) 73.097 2382 Rustic (Hoplodrina blanda) 73.099 2384 Vine's Rustic (Hoplodrina ambigua) 73.100 2391 Silky Wainscot (Chilodes maritima) 73.101 2380 Treble Lines (Charanyca trigrammica) 73.102 2302 Brown Rustic (Rusina ferruginea) 73.103 2392 Marsh Moth (Athetis pallustris) 73.104 2392a Porter's Rustic (Athetis hospes) 73.105 2301 Bird's Wing (Dypterygia scabriuscula) 73.106 2304 Orache Moth (Trachea atriplicis) 73.107 2300 Old Lady (Mormo maura) 73.109 2303 Straw Underwing (Thalpophila matura) 73.111 2097 Purple Cloud (Actinotia polyodon) 73.113 2306 Angle Shades (Phlogophora meticulosa) 73.114 2305 Small Angle Shades (Euplexia lucipara) 73.118 2367 Haworth's Minor (Celaena haworthii) 73.119 2368 Crescent (Helotropha leucostigma) 73.120 2352 Dusky Sallow (Eremobia ochroleuca) 73.121 2364 Frosted Orange (Gortyna flavago) 73.123 2361 Rosy Rustic (Hydraecia micacea) 73.124 2362 Butterbur (Hydraecia petasitis) 73.126 2358 Saltern Ear (Amphipoea fucosa) 73.127 2357 Large Ear (Amphipoea lucens) 73.128 2360 Ear Moth (Amphipoea oculea) 73.1281 2360x Ear Moth agg. (Amphipoea oculea agg.) 73.131 2353 Flounced Rustic (Luperina -

Records of Two New Palearctic Moth Species Associated with Queen Anne’S Lace in Nova Scotia
J. Acad. Entomol. Soc. 13: 54-57 (2017) NOTE Records of two new Palearctic moth species associated with Queen Anne’s lace in Nova Scotia Jeffrey Ogden Daucus carota Linnaeus, commonly known as Queen Anne’s lace or wild carrot, is native to Europe and southwestern Asia. It is believed to have been introduced to North America in soil ballast by the first European settlers (Lindroth 1957). It has historically been used as a food source and is the ancestral plant of our common cultivated garden carrot. Since its introduction, Daucus carota has spread widely and is common to much of North America. In Nova Scotia, this biennial is a very common plant of fields, roadsides and other weedy areas throughout the mainland and parts of Cape Breton (Zinck 1998). Previous insect surveys have recorded hundreds of species of insects attracted to the large white flower heads of the plant (Judd 1970; Largo & Mann 1987). Although considered a common weed species, Daucus carota is an important host to numerous beneficial insect species, including many pollinators and predatory species (Judd 1970). In this report, the first detections ofSitochroa palealis (Denis & Schiffermüller) (Lepidoptera: Crambidae), the carrot seed moth, and Depressaria depressana (Fabricius) (Lepidoptera: Depressariidae), the purple carrot seed moth, are described from Nova Scotia. Larvae of each species were collected within the flower heads of Daucus carota during the summers of 2015 to 2017. Adults were collected as part of an ongoing light trapping survey and through lab rearing. Verified photograph records were also considered. Voucher specimens have been deposited in the Nova Scotia Department of Natural Resources Reference Collection, the Nova Scotia Museum, and the author’s personal collection. -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties. -
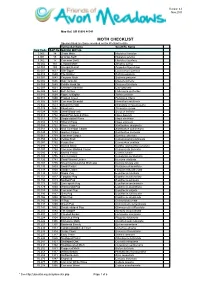
MOTH CHECKLIST Species Listed Are Those Recorded on the Wetland to Date
Version 4.0 Nov 2015 Map Ref: SO 95086 46541 MOTH CHECKLIST Species listed are those recorded on the Wetland to date. Vernacular Name Scientific Name New Code B&F No. MACRO MOTHS 3.005 14 Ghost Moth Hepialus humulae 3.001 15 Orange Swift Hepialus sylvina 3.002 17 Common Swift Hepialus lupulinus 50.002 161 Leopard Moth Zeuzera pyrina 54.008 169 Six-spot Burnet Zygaeba filipendulae 66.007 1637 Oak Eggar Lasiocampa quercus 66.010 1640 The Drinker Euthrix potatoria 68.001 1643 Emperor Moth Saturnia pavonia 65.002 1646 Oak Hook-tip Drepana binaria 65.005 1648 Pebble Hook-tip Drepana falcataria 65.007 1651 Chinese Character Cilix glaucata 65.009 1653 Buff Arches Habrosyne pyritoides 65.010 1654 Figure of Eighty Tethia ocularis 65.015 1660 Frosted Green Polyploca ridens 70.305 1669 Common Emerald Hermithea aestivaria 70.302 1673 Small Emerald Hemistola chrysoprasaria 70.029 1682 Blood-vein Timandra comae 70.024 1690 Small Blood-vein Scopula imitaria 70.013 1702 Small Fan-footed Wave Idaea biselata 70.011 1708 Single-dotted Wave Idaea dimidiata 70.016 1713 Riband Wave Idaea aversata 70.053 1722 Flame Carpet Xanthorhoe designata 70.051 1724 Red Twin-spot Carpet Xanthorhoe spadicearia 70.049 1728 Garden Carpet Xanthorhoe fluctuata 70.061 1738 Common Carpet Epirrhoe alternata 70.059 1742 Yellow Shell Camptogramma bilineata 70.087 1752 Purple Bar Cosmorhoe ocellata 70.093 1758 Barred Straw Eulithis (Gandaritis) pyraliata 70.097 1764 Common Marbled Carpet Chloroclysta truncata 70.085 1765 Barred Yellow Cidaria fulvata 70.100 1776 Green Carpet Colostygia pectinataria 70.126 1781 Small Waved Umber Horisme vitalbata 70.107 1795 November/Autumnal Moth agg Epirrita dilutata agg. -

Scottish Macro-Moth List, 2015
Notes on the Scottish Macro-moth List, 2015 This list aims to include every species of macro-moth reliably recorded in Scotland, with an assessment of its Scottish status, as guidance for observers contributing to the National Moth Recording Scheme (NMRS). It updates and amends the previous lists of 2009, 2011, 2012 & 2014. The requirement for inclusion on this checklist is a minimum of one record that is beyond reasonable doubt. Plausible but unproven species are relegated to an appendix, awaiting confirmation or further records. Unlikely species and known errors are omitted altogether, even if published records exist. Note that inclusion in the Scottish Invertebrate Records Index (SIRI) does not imply credibility. At one time or another, virtually every macro-moth on the British list has been reported from Scotland. Many of these claims are almost certainly misidentifications or other errors, including name confusion. However, because the County Moth Recorder (CMR) has the final say, dubious Scottish records for some unlikely species appear in the NMRS dataset. A modern complication involves the unwitting transportation of moths inside the traps of visiting lepidopterists. Then on the first night of their stay they record a species never seen before or afterwards by the local observers. Various such instances are known or suspected, including three for my own vice-county of Banffshire. Surprising species found in visitors’ traps the first time they are used here should always be regarded with caution. Clerical slips – the wrong scientific name scribbled in a notebook – have long caused confusion. An even greater modern problem involves errors when computerising the data. -

Lepidoptera: Pyraloidea) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Asselbergs, J. E. F.; Seguna, A.; Sammut, P. Recent records of Pyraloidea species new to Malta, including two species new to the European fauna (Lepidoptera: Pyraloidea) SHILAP Revista de Lepidopterología, vol. 36, núm. 144, diciembre, 2008, pp. 465-471 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45511220008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 465-471 Recent records of Pyral 15/12/08 16:37 Página 465 SHILAP Revta. lepid., 36 (144), diciembre 2008: 465-471 CODEN: SRLPEF ISSN:0300-5267 Recent records of Pyraloidea species new to Malta, including two species new to the European fauna (Lepidoptera: Pyraloidea) J. E. F. Asselbergs, A. Seguna & P. Sammut Abstract Acrobasis obliqua clusinella Zeller, 1848, Acrobasis centunculella (Mann, 1859), Psorosa dahliella (Treitschke, 1832), Psorosa mediterranella Amsel, 1953, Euzopherodes lutisignella (Mann, 1869), Delplanqueia inscriptella (Duponchel, 1836), Isauria dilucidella (Duponchel, 1836), Euchromius gozmanyi Bleszynski, 1961, Udea numeralis (Hübner, 1796), Ancylosis (Heterographis) costistrigella (Ragonot, 1890) and Caina deletella Ragonot, 1893 are new to the Maltese Islands. The latter two species are also the first records for the European fauna. Where known, data on the biology, the first stages and the distribution of the species is given. Additional notes on some other pyraloid species from Malta are also given.