Plankton Taxonomic and Size Diversity of Mediterranean Brackish Ponds in Spring: Influence of Abiotic and Biotic Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 296-78 WAC, Sawmills and Woodworking
Chapter 296-78 WAC Introduction Sawmills and Woodworking Operations _________________________________________________________________________________________________________ Chapter 296-78 WAC Sawmills and Woodworking Operations (Form Number F414-010-000) This book contains rules for Safety Standards for sawmills and woodworking operations, as adopted under the Washington Industrial Safety and Health Act of 1973 (Chapter 49.17 RCW). The rules in this book are effective March 2018. A brief promulgation history, set within brackets at the end of this chapter, gives statutory authority, administrative order of promulgation, and date of adoption of filing. TO RECEIVE E-MAIL UPDATES: Sign up at https://public.govdelivery.com/accounts/WADLI/subscriber/new?topic_id=WADLI_19 TO PRINT YOUR OWN PAPER COPY OR TO VIEW THE RULE ONLINE: Go to https://www.lni.wa.gov/safety-health/safety-rules/rules-by-chapter/?chapter=78/ DOSH CONTACT INFORMATION: Physical address: 7273 Linderson Way Tumwater, WA 98501-5414 (Located off I-5 Exit 101 south of Tumwater.) Mailing address: DOSH Standards and Information PO Box 44810 Olympia, WA 98504-4810 Telephone: 1-800-423-7233 For all L&I Contact information, visit https://www.lni.wa.gov/agency/contact/ Also available on the L&I Safety & Health website: DOSH Core Rules Other General Workplace Safety & Health Rules Industry and Task-Specific Rules Proposed Rules and Hearings Newly Adopted Rules and New Rule Information DOSH Directives (DD’s) See http://www.lni.wa.gov/Safety-Health/ Chapter 296-78 WAC Table of Contents Sawmills and Woodworking Operations _________________________________________________________________________________________________________ Chapter 296-78 WAC SAFETY STANDARDS FOR SAWMILLS AND WOODWORKING OPERATIONS WAC Page WAC 296-78-500 Foreword. -

Hult Reservoir Fish Species Composition, Size and Relative Abundance 2017
HULT RESERVOIR FISH SPECIES COMPOSITION, SIZE AND RELATIVE ABUNDANCE 2017 Prepared for BUREAU OF LAND MANAGEMENT SIUSLAW FIELD OFFICE 3106 Pierce Parkway, Suite E Springfield, Oregon 97477 i Prepared by Jeremy D. Romer Fred R. Monzyk Erik J. Suring Thomas A. Friesen Oregon Department of Fish and Wildlife Reservoir Research Project Corvallis Research Lab 28655 Highway 34 Corvallis, Oregon 97333 Cooperative Agreement: L12AC20634 February 2018 ii Table of Contents Summary ...................................................................................................................................................... 1 Background / Introduction ......................................................................................................................... 2 Methods ........................................................................................................................................................ 4 Fish Capture .............................................................................................................................................. 4 Water Chemistry ....................................................................................................................................... 5 Data Analysis ............................................................................................................................................. 5 Species Composition, Size, and Relative Abundance ................................................................................ 5 Coho Salmon and the -

Draft Program Environmental Impact Report
Draft Program Environmental Impact Report General Plan Amendment, Zone Reclassification, and Final Map Subdivision, Town of Scotia (State Clearinghouse No. 2007052042) Prepared for: The Pacific Lumber Company For submittal to: Humboldt County Department of Community Development Services Consulting Engineers and Geologists, Inc. 812 W. Wabash Ave. Eureka, CA 95501-2138 January 2008 707/441-8855 005161.106 Reference: 005161.106 Draft Program Environmental Impact Report General Plan Amendment, Zone Reclassification, and Final Map Subdivision, Town of Scotia (State Clearinghouse No. 2007052042) Prepared for: The Pacific Lumber Company Lead Agency: Humboldt County Department of Community Development Services, Planning Division Contact: Michael Wheeler, Senior Planner Humboldt County Planning 3015 H Street Eureka, CA 95501 (707) 268-3730 [email protected] Prepared by: Consulting Engineers and Geologists, Inc. 812 W. Wabash Ave. Eureka, CA 95501-2138 707-441-8855 January 2008 QA/QC: MKF___ G:\2005\005161_ScotiaMasterPlan\106_PEIR\rpt\Draft PEIR\Pub-Rev-DraftPEIR-rpt.doc Draft Program Environmental Impact Report Executive Summary General Plan Amendment, Zone Reclassification, and Final Map Subdivision Town of Scotia, January 2008 Executive Summary Introduction The Pacific Lumber Company (PALCO) has submitted a tentative map to Humboldt County Department of Community Development Services, Planning Division to subdivide the Town of Scotia. An additional application has been filed with the Local Agency Formation Commission (LAFCo) to form a Community Services District (CSD). Humboldt County is the lead agency under the California Environmental Quality Act (CEQA). The purpose of the subdivision is to create individual parcels for existing residential and commercial properties, and public facilities. The proposed subdivision would involve the sale of residential and commercial lots (all of which are currently owned and operated by PALCO) to individual property owners. -

JEMEZ MOUNTAIN RAILROADS April, 1990
JEMEZ MOUNTAIN RAILROADS Santa Fe National Forest New Mexico By Vernon J. Glover April, 1990 USDA Forest Service Southwestern Region Reprinted by Historical Society of New Mexico TABLE OF CONTENTS Cover: A rendering of the Santa Fe Northwestern's Locomotive Number 107. Originally purchased by the A&P, it served with the AT&SF until 1930 and then for the SFNW until 1942. FIGURES TABLES PUBLISHER'S NOTE ACKNOWLEDGEMENTS INTRODUCTION SANTA FE NORTHWESTERN RAILWAY The Cañon de San Diego Land Grant Building the Santa Fe Northwestern Railway Guadalupe Box Operating a Lumber Company Hard Times Reorganization New Mexico Lumber and Timber Company The Final Years CUBA EXTENSION RAILWAY Cuba Extension Railway San Juan Basin Railroad Santa Fe Northern Railroad Santa Fe, San Juan & Northern Railroad CONCLUSION APPENDIX A APPENDIX B REFERENCES CITED LIST OF FIGURES 1. Sidney Weil in August, 1956 2. General location map of the Cañon de San Diego Land Grant 3. American Hoist & Derrick Company log loader 4. Rio Grande trestle soon after its construction in early 1923 5. The sawmill at Bernalillo soon after its completion in 1924 6. Typical low pile trestle crossing an arroyo, circa 1923 7. Guadalupe Box during the railroad era 8. The large trestle leading to the Guadalupe Box tunnels 9. The southern approach to the Guadalupe Box 10. Map of the Jemez Mountain Railroads 11. A steel log car of the SFNW in the summer of 1939 at O'Neil Landing 12. Teams of horses were still used to skid logs in the woods in 1932 13. Locomotive Number 101 approaching the scene of a derailment 14. -

A Survey of Aquatic Habitats, Fishes and Other Aquatic Fauna of Elk Creek, Crescent City, California
A Survey of Aquatic Habitats, Fishes and other Aquatic Fauna of Elk Creek, Crescent City, California Justin M. Garwood ̶ Environmental Scientist Anadromous Fisheries Resource and Assessment January/2019 Program California Department of Fish and Wildlife 5341 Ericson Way, Arcata, CA 95521 Top Photo: Mainstem Elk Creek in the CDFW Elk Creek Wetland Wildlife Area; [email protected] Bottom Photo: Upper Elk Creek Estuary in the Spring at low tide 1 Background Elk Creek is a small (21.4 km2) coastal watershed that drains most of the greater Crescent City and Elk Valley coastal plain (Figure 1). Prior to this study, limited information on Elk Creek salmonid occupancy and distributions were summarized in Garwood (2012a). Information on Elk Creek Coho Salmon (Oncorhynchus kisutch) habitats and ecological threats are broadly covered in the Elk Creek chapter of the Southern Oregon Northern California Coho Salmon Recovery Plan (NOAA 2014), though specific details regarding local threats to salmonid populations remain unclear. In response to a need for more refined restoration planning in Elk Creek, the Smith River Alliance developed an initial restoration strategy for the watershed (SRA 2017). I initiated this study in 2013 to determine current occupancy status and spatial distributions of salmonids, among other species, occurring in Figure 1. Ariel photo of lower Elk Creek watershed looking west taken on April 7, 2016. Note the broad valley largely lacking human development and coniferous forest which is likely due to extensive wetland habitats. Much of the core watershed is buffered by dense stands of Coast Redwood and Sitka Spruce. Photo: J. -
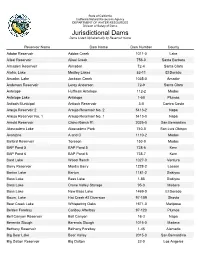
Jurisdictional Dams Listed Alphabetically by Reservoir Name
State of California California Natural Resources Agency DEPARTMENT OF WATER RESOURCES Division of Safety of Dams Jurisdictional Dams Dams Listed Alphabetically by Reservoir Name Reservoir Name Dam Name Dam Number County Adobe Reservoir Adobe Creek 1011-0 Lake Alisal Reservoir Alisal Creek 756-0 Santa Barbara Almaden Reservoir Almaden 72-4 Santa Clara Aloha, Lake Medley Lakes 53-11 El Dorado Amador, Lake Jackson Creek 1035-0 Amador Anderson Reservoir Leroy Anderson 72-9 Santa Clara Antelope Huffman Antelope 112-2 Modoc Antelope Lake Antelope 1-50 Plumas Antioch Municipal Antioch Reservoir 3-0 Contra Costa Araujo Reservoir 2 Araujo Reservoir No. 2 5413-2 Napa Araujo Reservoir No. 1 Araujo Reservoir No. 1 5413-0 Napa Arnold Reservoir Chino Ranch #1 2025-0 San Bernardino Atascadero Lake Atascadero Park 740-0 San Luis Obispo Avanzino A and C 1110-2 Modoc Ballard Reservoir Toreson 153-0 Modoc BAP Pond 5 BAP Pond 5 738-6 Kern BAP Pond 6 BAP Pond 6 738-7 Kern Bard Lake Wood Ranch 1027-0 Ventura Barry Reservoir Mardis Barry 1228-2 Lassen Barton Lake Barton 1181-2 Siskiyou Bass Lake Bass Lake 1-85 Siskiyou Bass Lake Crane Valley Storage 95-3 Madera Bass Lake New Bass Lake 1469-0 El Dorado Baum, Lake Hat Creek #2 Diversion 97-109 Shasta Bear Creek Lake Whispering Oaks 1671-0 Mariposa Belden Forebay Caribou Afterbay 97-120 Plumas Bell Canyon Reservoir Bell Canyon 16-3 Napa Berenda Slough Berenda Slough 1015-0 Madera Bethany Reservoir Bethany Forebay 1-45 Alameda Big Bear Lake Bear Valley 2015-0 San Bernardino Big Dalton Reservoir Big Dalton 32-0 Los Angeles Jurisdictional Dams Dams Listed Alphabetically by Reservoir Name Page 2 of 12 Reservoir Name Dam Name Dam Number County Big Pine Lake Big Pine Creek 6-11 Inyo Big Reservoir Morning Star 325-0 Placer Big Sage Reservoir Big Sage 55-0 Modoc Big Tujunga Reservoir Big Tujunga No. -

Reservation Narrow Gauge
BlLES - COLEMAN LUMBER COMPANY'S RESERVATION NARROW GAUGE The Last Northwest/Washington State Narrow Gauge Logging Rai I road 1921 - 1948 ..... With a Supplement on the DIAMOND MATCH COMPANY PRIEST LAKE RAILROAD The West '8 Most Modern Narrow Gauge Loggi ng Rail road BY JOHN E. LEWI S l ~~'t1.0N NARRO~ ~i>~ Q~b: ~i> A Chronicle Of ~~ BILES-COLEMAN LUMBER COMPANY'S NARROW GAUGE OA... " ..~4l( t\O~ ()Jo,,-, CREEK Rl\1.~\~f.") at 4RROW SHORtt The LAST NARROW GAUGE LOGGING RAILROAD In Washington State First Edition THI SIS OF 2500 BOOK NO. PRINTED By JOHN E. LEWIS © 1980 by John Edward Lewis All rights reserved, including those to reproduce this book, or parts thereof in any form without permission in writing from the publisher. FIRST EDITION Library of Congress Catalog Card Number: 80-80621 PUBLISHER'S NOTE ... Scale equipment drawings used in this book are based upon actual manu facturer's erection drawings (log cars, speeder, Shay, and 2-6-6-2), equipment of the same size, class and approximate year of construction (Heisler), or actual equip me.nt item (log loader) . Any corrections/ additions forwarded by readers will be gratefully accepted by the authol·. Although it seems certain that color photos of the Biles-Coleman line or its equipmellt were taken by some of the thousands of workers engaged in building the nearby Grand Coulee Dam Project, a search over the years has failed to locate any such photos of this type. If forwarded to the author, together with other per tinent corrections/ additions will be included in a revised second edition should de mand warrant. -

Waste Stabilisation Ponds Is the Third Volume in the Series Biological Wastewater Treatment
TREATMENT SERIES TREATMENT BIOLOGICAL WASTEWATER BIOLOGICAL WASTEWATER BIOLOGICAL WASTEWATER TREATMENT SERIES VOLUME 3 Waste Stabilisation Ponds is the third volume in the series Biological Wastewater Treatment. The major variants of pond systems are fully covered, namely: VOLUME 3 • facultative ponds • anaerobic ponds • aerated lagoons and WASTE • maturation ponds. WASTE STABILISATION PONDS STABILISATION WASTE The book presents in a clear and didactic way the main concepts, working STABILISATION principles, expected removal efficiencies, design criteria, design examples, construction aspects, operational guidelines and sludge management for pond systems. PONDS The Biological Wastewater Treatment series is based on the book Biological Wastewater Treatment in Warm Climate Regions and on a highly acclaimed set of best selling textbooks. This international version is comprised by six textbooks giving a state-of-the-art presentation of the science and technology of biological wastewater treatment. Books in the Biological Wastewater Treatment series are: • Volume 1: Wastewater Characteristics, Treatment and Disposal • Volume 2: Basic Principles of Wastewater Treatment • Volume 3: Waste Stabilisation Ponds • Volume 4: Anaerobic Reactors • Volume 5: Activated Sludge and Aerobic Biofilm Reactors • Volume 6: Sludge Treatment and Disposal Marcos von Sperling Marcos 1843391635 Marcos von Sperling Downloaded from http://iwaponline.com/ebooks/book-pdf/1112/wio9781780402109.pdf by guest on 01 October 2020 6.14 x 9.21.375 6.14 x 9.21 Waste Stabilisation Ponds Downloaded from http://iwaponline.com/ebooks/book-pdf/1112/wio9781780402109.pdf by guest on 01 October 2020 Biological Wastewater Treatment Series The Biological Wastewater Treatment series is based on the book Biological Wastewater Treatment in Warm Climate Regions and on a highly acclaimed set of best selling textbooks. -

Timber Products Processing Effluent Guidelines
RULES AND REGULATIONS Title 40-Protection of the Environment Both of these documents were made (3) Commenters said that the disposal CHAPTER I-ENVIRONMENTAL available to the public and circulated to of process waste water into a log pond or PROTECTION AGENCY interested persons at approximately the mill pond, If available would be a practi- time of publication of the notice of pro- cal method of control. SUBCHAPTER N-EFFLUENT GUIDELINES AND STANDARDS posed rulemaking. The regulations promulgated here ex- nterested persons were invited to par-.. clude those facilities that include wet PART 429-TIMBER PRODUCTS PROC- ticipate in the rulemaking by submitting storage and/or handling or part of this ESSING POINT SOURCE CATEGORY written comments within 30 days from normal operating practice. Further data On January 3, 1974, notice was pub- the date of publication. Prior public par- is being developed, and guidelines and lished in the FEDERAL REGISTER (39 FR ticipation in the form of solicited com- standards for these facilities will be es- 938), that the Environmental Protection ments and responses from the States, tablished at a later date. For wet storage Agency (EPA or Agency) was proposing Federal agencies, and other interested facilities the disposal of process waste effluent limitations guidelines for exist- parties were described in the preamble water into a log pond or mill pond Is one ing sources and standards of perform- to the proposed regulation. The EPA has method of control. It should be noted that ance and pretreatment standards for considered carefully all of the comments the Development Document provides In- new sources within the barking, veneer, received and a discussion of these com- formation to show that with reasonable plywood, hardboard-dry process, hard- ments with the Agency's response there- unit op:eration and process management board-wet process, wood preserving, to follows, individual unit operations within the wood preserving-steam and wood pre- (a) Summary of comments. -

Stormwater Management Program for the City of Newark, Ohio April 2009
City of Newark, Ohio FINAL REPORT Stormwater Management Program for the City of Newark, Ohio April 2009 Report Prepared For: City of Newark 40 West Main Street Newark, Ohio 43055 0821249 INDEPENDENT ENVIRONMENTAL ENGINEERS, SCIENTISTS AND CONSULTANTS Introduction The City of Newark (City) is located in central Licking County, Ohio, and encompasses approximately 19 square miles (12,300 acres) of land. The City is a component of the Licking County Urban Area (UA), and, according to the 2000 Census, has a population of approximately 46,279 people. While the majority of the property inside the corporation limits of Newark is identified as part of the UA, some City property lies outside of the UA boundary. To provide continuity, the City considered the entire land area within the city limits when initially developing the stormwater management program (SWMP). The City lies within the greater Muskingum River watershed and the Licking River sub-basin (HUC 05040006). Primary receiving waters include Raccoon Creek, North Fork of the Licking River, South Fork of the Licking River, Licking River and Log Pond Run. Ohio Environmental Protection Agency (Ohio EPA) performed field work in 1993 in the Licking River basin and summarized associated findings in a report entitled Biological and Water Quality Study of the Licking River and Selected Tributaries. Results indicated that the Licking River fully attained warmwater habitat (WWH) aquatic life use except immediately downstream of Dillon Lake, which lies south of the City of Newark in Muskingum County. In the summer of 2008, Ohio EPA began a new study of the Licking River watershed as a first step toward creating a Total Maximum Daily Load (TMDL). -

Waste Stabilisation Ponds Is the Third Volume in the Series Biological Wastewater Treatment
TREATMENT SERIES TREATMENT BIOLOGICAL WASTEWATER BIOLOGICAL WASTEWATER BIOLOGICAL WASTEWATER TREATMENT SERIES VOLUME 3 Waste Stabilisation Ponds is the third volume in the series Biological Wastewater Treatment. The major variants of pond systems are fully covered, namely: VOLUME 3 • facultative ponds • anaerobic ponds • aerated lagoons and WASTE • maturation ponds. WASTE STABILISATION PONDS STABILISATION WASTE The book presents in a clear and didactic way the main concepts, working STABILISATION principles, expected removal efficiencies, design criteria, design examples, construction aspects, operational guidelines and sludge management for pond systems. PONDS The Biological Wastewater Treatment series is based on the book Biological Wastewater Treatment in Warm Climate Regions and on a highly acclaimed set of best selling textbooks. This international version is comprised by six textbooks giving a state-of-the-art presentation of the science and technology of biological wastewater treatment. Books in the Biological Wastewater Treatment series are: • Volume 1: Wastewater Characteristics, Treatment and Disposal • Volume 2: Basic Principles of Wastewater Treatment • Volume 3: Waste Stabilisation Ponds • Volume 4: Anaerobic Reactors • Volume 5: Activated Sludge and Aerobic Biofilm Reactors • Volume 6: Sludge Treatment and Disposal Marcos von Sperling Marcos 1843391635 Marcos von Sperling 6.14 x 9.21.375 6.14 x 9.21 Waste Stabilisation Ponds Biological Wastewater Treatment Series The Biological Wastewater Treatment series is based on the -

43230 Page 1 of 8 Pages NATIONAL POLLUTANT DISCHARGE
Fik ,umber: 43230 Page 1 of 8 Pages NATIONAL POLLUTANT DISCHARGE ELIMINATION SYSTEM WASTE DISCHARGE PERMIT REVIEW REPORT Department of Environmental Quality 2146 NE 4th Street, Suite 104 Bend, OR 97701 Telephone: (541) 388-6146 Issued pursuant to ORS 468B.050 and The Federal Clean Water Act ISSUED TO: SOURCES COVERED BY THIS PERMIT: JELD-WEN, inc. Outfall Outfall P.O. Box 1329 Type of Waste Number Location Klamath Falls, OR 97601 Non-contact Cooling Water 001 Klamath Lake Boiler Blowdown, and Storm Water Runoff Storm Water Runoff 003 Klamath Lake Log Deck Sprinkling Water 004 Retention Pond PLANT TYPE AND LOCATION: RECEIVING SYSTEM INFORMATION: Wood Products Manufacturing Plant Basin: Klamath 3922 Lakeport Boulevard Sub-Basin: Upper Klamath Klamath Falls, Oregon Receiving Stream: Upper Klamath Lake LIDD 1218255422538-255-D County: Klamath EPA REFERENCE NO: OR-003101-1 Background: JELD-WEN, inc. (JWi) operates a wood products manufacturing facility north of Klamath Falls, Oregon along the shore of Upper Klamath Lake. At the time this document was drafted, the facility had 530 employees. The facility consists of a sawmill, planer mill, door plant, door skin manufacturing plant, and cutstock. The Company makes doors, door and window parts, door skins and lumber. The facility operates up to seven days a week with up to three shifts per day. Non-process wastewater from the JWi plant discharges into two elongated storage ponds. The second pond discharges into the Upper Klamath Lake at Outfall No. 001. The ponds are also used for settling and conveying storm water from onsite and offsite sources. The wastewater in the ponds may be used for irrigation in Department approved areas, log deck sprinkling, and/or for fire protection, as needed.