UV-Irradiation- and Inflammation-Induced Skin Barrier Dysfunction Is Associated with the Expression of Olfactory Receptor Genes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genetic Variation Across the Human Olfactory Receptor Repertoire Alters Odor Perception
bioRxiv preprint doi: https://doi.org/10.1101/212431; this version posted November 1, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Genetic variation across the human olfactory receptor repertoire alters odor perception Casey Trimmer1,*, Andreas Keller2, Nicolle R. Murphy1, Lindsey L. Snyder1, Jason R. Willer3, Maira Nagai4,5, Nicholas Katsanis3, Leslie B. Vosshall2,6,7, Hiroaki Matsunami4,8, and Joel D. Mainland1,9 1Monell Chemical Senses Center, Philadelphia, Pennsylvania, USA 2Laboratory of Neurogenetics and Behavior, The Rockefeller University, New York, New York, USA 3Center for Human Disease Modeling, Duke University Medical Center, Durham, North Carolina, USA 4Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, North Carolina, USA 5Department of Biochemistry, University of Sao Paulo, Sao Paulo, Brazil 6Howard Hughes Medical Institute, New York, New York, USA 7Kavli Neural Systems Institute, New York, New York, USA 8Department of Neurobiology and Duke Institute for Brain Sciences, Duke University Medical Center, Durham, North Carolina, USA 9Department of Neuroscience, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA *[email protected] ABSTRACT The human olfactory receptor repertoire is characterized by an abundance of genetic variation that affects receptor response, but the perceptual effects of this variation are unclear. To address this issue, we sequenced the OR repertoire in 332 individuals and examined the relationship between genetic variation and 276 olfactory phenotypes, including the perceived intensity and pleasantness of 68 odorants at two concentrations, detection thresholds of three odorants, and general olfactory acuity. -

SNP Genotypes of Olfactory Receptor Genes Associated with Olfactory Ability in German Shepherd Dogs
SHORT COMMUNICATION doi: 10.1111/age.12389 SNP genotypes of olfactory receptor genes associated with olfactory ability in German Shepherd dogs † ‡ – M. Yang*, G.-J. Geng , W. Zhang , L. Cui§, H.-X. Zhang and J.-L. Zheng‡ † *Police-dog Technology Department, National Police University of China, Shenyang, Liaoning 110034, China. Technology Department, ‡ Shenyang Traffic Police Detachment, Shenyang, Liaoning 110001, China. Forensic Medicine Department, National Police University of China, Shenyang, Liaoning 110854, China. §Document Inspection Department, National Police University of China, Shenyang, Liaoning – 110854, China. Mark Inspection Department, National Police University of China, Shenyang, Liaoning 110854, China. Summary To find out the relationship between SNP genotypes of canine olfactory receptor genes and olfactory ability, 28 males and 20 females from German Shepherd dogs in police service were scored by odor detection tests and analyzed using the Beckman GenomeLab SNPstream. The representative 22 SNP loci from the exonic regions of 12 olfactory receptor genes were investigated, and three kinds of odor (human, ice drug and trinitrotoluene) were detected. The results showed that the SNP genotypes at the OR10H1-like:c.632C>T, OR10H1-like:c.770A>T, OR2K2-like:c.518G>A, OR4C11-like: c.511T>G and OR4C11-like:c.692G>A loci had a statistically significant effect on the scenting abilities (P < 0.001). The kind of odor influenced the performances of the dogs (P < 0.001). In addition, there were interactions between genotype and the kind of odor at the following loci: OR10H1-like:c.632C>T, OR10H1-like:c.770A>T, OR4C11-like:c.511T>G and OR4C11-like:c.692G>A(P< 0.001). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

LETTER Doi:10.1038/Nature09515
LETTER doi:10.1038/nature09515 Distant metastasis occurs late during the genetic evolution of pancreatic cancer Shinichi Yachida1*, Siaˆn Jones2*, Ivana Bozic3, Tibor Antal3,4, Rebecca Leary2, Baojin Fu1, Mihoko Kamiyama1, Ralph H. Hruban1,5, James R. Eshleman1, Martin A. Nowak3, Victor E. Velculescu2, Kenneth W. Kinzler2, Bert Vogelstein2 & Christine A. Iacobuzio-Donahue1,5,6 Metastasis, the dissemination and growth of neoplastic cells in an were present in the primary pancreatic tumours from which the meta- organ distinct from that in which they originated1,2, is the most stases arose. A small number of these samples of interest were cell lines common cause of death in cancer patients. This is particularly true or xenografts, similar to the index lesions, whereas the majority were for pancreatic cancers, where most patients are diagnosed with fresh-frozen tissues that contained admixed neoplastic, stromal, metastatic disease and few show a sustained response to chemo- inflammatory, endothelial and normal epithelial cells (Fig. 1a). Each therapy or radiation therapy3. Whether the dismal prognosis of tissue sample was therefore microdissected to minimize contaminat- patients with pancreatic cancer compared to patients with other ing non-neoplastic elements before purifying DNA. types of cancer is a result of late diagnosis or early dissemination of Two categories of mutations were identified (Fig. 1b). The first and disease to distant organs is not known. Here we rely on data gen- largest category corresponded to those mutations present in all samples erated by sequencing the genomes of seven pancreatic cancer meta- from a given patient (‘founder’ mutations, mean of 64%, range 48–83% stases to evaluate the clonal relationships among primary and of all mutations per patient; Fig. -

Supplementary Methods
Heterogeneous Contribution of Microdeletions in the Development of Common Generalized and Focal epilepsies. SUPPLEMENTARY METHODS Epilepsy subtype extended description. Genetic Gereralized Epilepsy (GGE): Features unprovoked tonic and/or clonic seizures, originated inconsistently at some focal point within the brain that rapidly generalizes engaging bilateral distributed spikes and waves discharges on the electroencephalogram. This generalization can include cortical and sub cortical structures but not necessarily the entire cortex[1]. GGE is the most common group of epilepsies accounting for 20% of all cases[2]. It is characterized by an age-related onset and a strong familial aggregation and heritability which allows the assumption of a genetic cause. Although genetic associations have been identified, a broad spectrum of causes is acknowledged and remains largely unsolved [3]. Rolandic Epilepsy (RE): Commonly known also as Benign Epilepsy with Centrotemporal Spikes (BECTS), hallmarks early onset diagnosis (mean onset = 7 years old) with brief, focal hemifacial or oropharyngeal sensorimotor seizures alongside speech arrest and secondarily generalized tonic– clonic seizures, which mainly occur during sleep[4]. Rolandic epilepsy features a broad spectrum of less benign related syndromes called atypical Rolandic epilepsy (ARE), including benign partial epilepsy (ABPE), Landau–Kleffner syndrome(LKS) and epileptic encephalopathy with continuous spike-and-waves during sleep (CSWSS)[5]. Together they are the most common childhood epilepsy with a prevalence of 0.2–0.73/1000 (i.e. _1/2500)[6]. Adult Focal Epilepsy (AFE). Focal epilepsy is characterized by sporadic events of seizures originated within a specific brain region and restricted to one hemisphere. Although they can exhibit more than one network of wave discharges on the electroencephalogram, and different degrees of spreading, they feature a consistent site of origin. -

Deep Sequencing of the Human Retinae Reveals the Expression of Odorant Receptors
fncel-11-00003 January 20, 2017 Time: 14:24 # 1 CORE Metadata, citation and similar papers at core.ac.uk Provided by Frontiers - Publisher Connector ORIGINAL RESEARCH published: 24 January 2017 doi: 10.3389/fncel.2017.00003 Deep Sequencing of the Human Retinae Reveals the Expression of Odorant Receptors Nikolina Jovancevic1*, Kirsten A. Wunderlich2, Claudia Haering1, Caroline Flegel1, Désirée Maßberg1, Markus Weinrich1, Lea Weber1, Lars Tebbe2, Anselm Kampik3, Günter Gisselmann1, Uwe Wolfrum2, Hanns Hatt1† and Lian Gelis1† 1 Department of Cell Physiology, Ruhr-University Bochum, Bochum, Germany, 2 Department of Cell and Matrix Biology, Johannes Gutenberg University of Mainz, Mainz, Germany, 3 Department of Ophthalmology, Ludwig Maximilian University of Munich, Munich, Germany Several studies have demonstrated that the expression of odorant receptors (ORs) occurs in various tissues. These findings have served as a basis for functional studies that demonstrate the potential of ORs as drug targets for a clinical application. To the best of our knowledge, this report describes the first evaluation of the mRNA expression of ORs and the localization of OR proteins in the human retina that set a Edited by: stage for subsequent functional analyses. RNA-Sequencing datasets of three individual Hansen Wang, University of Toronto, Canada neural retinae were generated using Next-generation sequencing and were compared Reviewed by: to previously published but reanalyzed datasets of the peripheral and the macular Ewald Grosse-Wilde, human retina and to reference tissues. The protein localization of several ORs was Max Planck Institute for Chemical Ecology (MPG), Germany investigated by immunohistochemistry. The transcriptome analyses detected an average Takaaki Sato, of 14 OR transcripts in the neural retina, of which OR6B3 is one of the most highly National Institute of Advanced expressed ORs. -

An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors
Ecology and Evolutionary Biology 2021; 6(3): 53-77 http://www.sciencepublishinggroup.com/j/eeb doi: 10.11648/j.eeb.20210603.11 ISSN: 2575-3789 (Print); ISSN: 2575-3762 (Online) An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors Miguel Angel Fuertes*, Carlos Alonso Department of Microbiology, Centre for Molecular Biology “Severo Ochoa”, Spanish National Research Council and Autonomous University, Madrid, Spain Email address: *Corresponding author To cite this article: Miguel Angel Fuertes, Carlos Alonso. An Evolutionary Based Strategy for Predicting Rational Mutations in G Protein-Coupled Receptors. Ecology and Evolutionary Biology. Vol. 6, No. 3, 2021, pp. 53-77. doi: 10.11648/j.eeb.20210603.11 Received: April 24, 2021; Accepted: May 11, 2021; Published: July 13, 2021 Abstract: Capturing conserved patterns in genes and proteins is important for inferring phenotype prediction and evolutionary analysis. The study is focused on the conserved patterns of the G protein-coupled receptors, an important superfamily of receptors. Olfactory receptors represent more than 2% of our genome and constitute the largest family of G protein-coupled receptors, a key class of drug targets. As no crystallographic structures are available, mechanistic studies rely on the use of molecular dynamic modelling combined with site-directed mutagenesis data. In this paper, we hypothesized that human-mouse orthologs coding for G protein-coupled receptors maintain, at speciation events, shared compositional structures independent, to some extent, of their percent identity as reveals a method based in the categorization of nucleotide triplets by their gross composition. The data support the consistency of the hypothesis, showing in ortholog G protein-coupled receptors the presence of emergent shared compositional structures preserved at speciation events. -

The Odorant Receptor OR2W3 on Airway Smooth Muscle Evokes Bronchodilation Via a Cooperative Chemosensory Tradeoff Between TMEM16A and CFTR
The odorant receptor OR2W3 on airway smooth muscle evokes bronchodilation via a cooperative chemosensory tradeoff between TMEM16A and CFTR Jessie Huanga,1,2, Hong Lama,1, Cynthia Koziol-Whiteb,c, Nathachit Limjunyawongd, Donghwa Kime, Nicholas Kimb, Nikhil Karmacharyac, Premraj Rajkumarf, Danielle Firera, Nicholas M. Dalesiog, Joseph Judec, Richard C. Kurtenh, Jennifer L. Pluznickf, Deepak A. Deshpandei, Raymond B. Penni, Stephen B. Liggette,j, Reynold A. Panettieri Jrc, Xinzhong Dongd,k, and Steven S. Anb,c,2 aDepartment of Environmental Health and Engineering, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD 21205; bDepartment of Pharmacology, Rutgers-Robert Wood Johnson Medical School, The State University of New Jersey, Piscataway, NJ 08854; cRutgers Institute for Translational Medicine and Science, New Brunswick, NJ 08901; dSolomon H. Snyder Department of Neuroscience, The Johns Hopkins University School of Medicine, Baltimore, MD 21205; eCenter for Personalized Medicine, Morsani College of Medicine, University of South Florida, Tampa, FL 33612; fDepartment of Physiology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205; gDepartment of Anesthesiology and Critical Care Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD 21205; hDepartment of Physiology and Biophysics, University of Arkansas for Medical Sciences, Little Rock, AR 72205; iDivision of Pulmonary and Critical Care Medicine, Department of Medicine, Center for Translational Medicine, Jane and Leonard Korman -

Misexpression of Cancer/Testis (Ct) Genes in Tumor Cells and the Potential Role of Dream Complex and the Retinoblastoma Protein Rb in Soma-To-Germline Transformation
Michigan Technological University Digital Commons @ Michigan Tech Dissertations, Master's Theses and Master's Reports 2019 MISEXPRESSION OF CANCER/TESTIS (CT) GENES IN TUMOR CELLS AND THE POTENTIAL ROLE OF DREAM COMPLEX AND THE RETINOBLASTOMA PROTEIN RB IN SOMA-TO-GERMLINE TRANSFORMATION SABHA M. ALHEWAT Michigan Technological University, [email protected] Copyright 2019 SABHA M. ALHEWAT Recommended Citation ALHEWAT, SABHA M., "MISEXPRESSION OF CANCER/TESTIS (CT) GENES IN TUMOR CELLS AND THE POTENTIAL ROLE OF DREAM COMPLEX AND THE RETINOBLASTOMA PROTEIN RB IN SOMA-TO- GERMLINE TRANSFORMATION", Open Access Master's Thesis, Michigan Technological University, 2019. https://doi.org/10.37099/mtu.dc.etdr/933 Follow this and additional works at: https://digitalcommons.mtu.edu/etdr Part of the Cancer Biology Commons, and the Cell Biology Commons MISEXPRESSION OF CANCER/TESTIS (CT) GENES IN TUMOR CELLS AND THE POTENTIAL ROLE OF DREAM COMPLEX AND THE RETINOBLASTOMA PROTEIN RB IN SOMA-TO-GERMLINE TRANSFORMATION By Sabha Salem Alhewati A THESIS Submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE In Biological Sciences MICHIGAN TECHNOLOGICAL UNIVERSITY 2019 © 2019 Sabha Alhewati This thesis has been approved in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE in Biological Sciences. Department of Biological Sciences Thesis Advisor: Paul Goetsch. Committee Member: Ebenezer Tumban. Committee Member: Zhiying Shan. Department Chair: Chandrashekhar Joshi. Table of Contents List of figures .......................................................................................................................v -

WO 2019/068007 Al Figure 2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/068007 Al 04 April 2019 (04.04.2019) W 1P O PCT (51) International Patent Classification: (72) Inventors; and C12N 15/10 (2006.01) C07K 16/28 (2006.01) (71) Applicants: GROSS, Gideon [EVIL]; IE-1-5 Address C12N 5/10 (2006.0 1) C12Q 1/6809 (20 18.0 1) M.P. Korazim, 1292200 Moshav Almagor (IL). GIBSON, C07K 14/705 (2006.01) A61P 35/00 (2006.01) Will [US/US]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., C07K 14/725 (2006.01) P.O. Box 4044, 7403635 Ness Ziona (TL). DAHARY, Dvir [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (21) International Application Number: Box 4044, 7403635 Ness Ziona (IL). BEIMAN, Merav PCT/US2018/053583 [EilL]; c/o ImmPACT-Bio Ltd., 2 Ilian Ramon St., P.O. (22) International Filing Date: Box 4044, 7403635 Ness Ziona (E.). 28 September 2018 (28.09.2018) (74) Agent: MACDOUGALL, Christina, A. et al; Morgan, (25) Filing Language: English Lewis & Bockius LLP, One Market, Spear Tower, SanFran- cisco, CA 94105 (US). (26) Publication Language: English (81) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of national protection available): AE, AG, AL, AM, 62/564,454 28 September 2017 (28.09.2017) US AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 62/649,429 28 March 2018 (28.03.2018) US CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (71) Applicant: IMMP ACT-BIO LTD. -

Sean Raspet – Molecules
1. Commercial name: Fructaplex© IUPAC Name: 2-(3,3-dimethylcyclohexyl)-2,5,5-trimethyl-1,3-dioxane SMILES: CC1(C)CCCC(C1)C2(C)OCC(C)(C)CO2 Molecular weight: 240.39 g/mol Volume (cubic Angstroems): 258.88 Atoms number (non-hydrogen): 17 miLogP: 4.43 Structure: Biological Properties: Predicted Druglikenessi: GPCR ligand -0.23 Ion channel modulator -0.03 Kinase inhibitor -0.6 Nuclear receptor ligand 0.15 Protease inhibitor -0.28 Enzyme inhibitor 0.15 Commercial name: Fructaplex© IUPAC Name: 2-(3,3-dimethylcyclohexyl)-2,5,5-trimethyl-1,3-dioxane SMILES: CC1(C)CCCC(C1)C2(C)OCC(C)(C)CO2 Predicted Olfactory Receptor Activityii: OR2L13 83.715% OR1G1 82.761% OR10J5 80.569% OR2W1 78.180% OR7A2 77.696% 2. Commercial name: Sylvoxime© IUPAC Name: N-[4-(1-ethoxyethenyl)-3,3,5,5tetramethylcyclohexylidene]hydroxylamine SMILES: CCOC(=C)C1C(C)(C)CC(CC1(C)C)=NO Molecular weight: 239.36 Volume (cubic Angstroems): 252.83 Atoms number (non-hydrogen): 17 miLogP: 4.33 Structure: Biological Properties: Predicted Druglikeness: GPCR ligand -0.6 Ion channel modulator -0.41 Kinase inhibitor -0.93 Nuclear receptor ligand -0.17 Protease inhibitor -0.39 Enzyme inhibitor 0.01 Commercial name: Sylvoxime© IUPAC Name: N-[4-(1-ethoxyethenyl)-3,3,5,5tetramethylcyclohexylidene]hydroxylamine SMILES: CCOC(=C)C1C(C)(C)CC(CC1(C)C)=NO Predicted Olfactory Receptor Activity: OR52D1 71.900% OR1G1 70.394% 0R52I2 70.392% OR52I1 70.390% OR2Y1 70.378% 3. Commercial name: Hyperflor© IUPAC Name: 2-benzyl-1,3-dioxan-5-one SMILES: O=C1COC(CC2=CC=CC=C2)OC1 Molecular weight: 192.21 g/mol Volume -
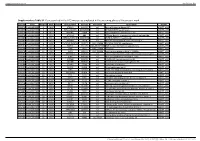
Supplementary Table S1. Genes Printed in the HC5 Microarray Employed in the Screening Phase of the Present Work
Supplementary material Ann Rheum Dis Supplementary Table S1. Genes printed in the HC5 microarray employed in the screening phase of the present work. CloneID Plate Position Well Length GeneSymbol GeneID Accession Description Vector 692672 HsxXG013989 2 B01 STK32A 202374 null serine/threonine kinase 32A pANT7_cGST 692675 HsxXG013989 3 C01 RPS10-NUDT3 100529239 null RPS10-NUDT3 readthrough pANT7_cGST 692678 HsxXG013989 4 D01 SPATA6L 55064 null spermatogenesis associated 6-like pANT7_cGST 692679 HsxXG013989 5 E01 ATP1A4 480 null ATPase, Na+/K+ transporting, alpha 4 polypeptide pANT7_cGST 692689 HsxXG013989 6 F01 ZNF816-ZNF321P 100529240 null ZNF816-ZNF321P readthrough pANT7_cGST 692691 HsxXG013989 7 G01 NKAIN1 79570 null Na+/K+ transporting ATPase interacting 1 pANT7_cGST 693155 HsxXG013989 8 H01 TNFSF12-TNFSF13 407977 NM_172089 TNFSF12-TNFSF13 readthrough pANT7_cGST 693161 HsxXG013989 9 A02 RAB12 201475 NM_001025300 RAB12, member RAS oncogene family pANT7_cGST 693169 HsxXG013989 10 B02 SYN1 6853 NM_133499 synapsin I pANT7_cGST 693176 HsxXG013989 11 C02 GJD3 125111 NM_152219 gap junction protein, delta 3, 31.9kDa pANT7_cGST 693181 HsxXG013989 12 D02 CHCHD10 400916 null coiled-coil-helix-coiled-coil-helix domain containing 10 pANT7_cGST 693184 HsxXG013989 13 E02 IDNK 414328 null idnK, gluconokinase homolog (E. coli) pANT7_cGST 693187 HsxXG013989 14 F02 LYPD6B 130576 null LY6/PLAUR domain containing 6B pANT7_cGST 693189 HsxXG013989 15 G02 C8orf86 389649 null chromosome 8 open reading frame 86 pANT7_cGST 693194 HsxXG013989 16 H02 CENPQ 55166