Diets of Double-Crested Cormorants in the Lake Winnebago System, Wisconsin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Lower Fox River Lake - Boat Landings
Lower Fox River Lake - Boat Landings Little Lake Butte des Morts Boat Landing Details Map Lake Butte Des Morts -- Ninth Street Boat Launch Details Google Map Little Lake Butte Des Morts -- Menasha - Fritze Park Access Details Google Map Winnebago Pool Lakes - Boat Landings Lake Winnebago Boat Landings Details Map Lake Winnebago -- Brothertown Harbor, Off Cty H Details Google Map Lake Winnebago -- Oshkosh - Otter Street/ Ceape Ave - Carry-In Access Details Google Map Lake Winnebago -- Vinland - Grundman Park - Osh-O-Nee Details Google Map Lake Winnebago – Fisherman’s Park / Taycheedah Details Google Map Lake Winnebago -- Calumet County Boat Launch Details Google Map Lake Winnebago -- Oshkosh - Doemel Point Access - Murdock & Menominee Details Google Map Lake Winnebago -- Oshkosh - Fugleburg Park Details Google Map Lake Winnebago -- Oshkosh - 24th Street Access Details Google Map Lake Winnebago -- Calumet - Columbia Park Details Google Map Lake Winnebago -- Oshkosh - Mill Street Access Details Google Map Lake Winnebago -- Oshkosh - Menominee Park Details Google Map Lake Winnebago -- Black Wolf - Nagy Park Landing, Hwy 45 Details Google Map Lake Winnebago -- Fond Du Lac - Lakeside West Park - Supple Marsh Details Google Map Lake Winnebago -- Sherwood - High Cliff State Park Details Google Map Lake Winnebago -- Columbia Park Boat Access - Google Map Lake Winnebago -- Neenah - South Park Ave., Rec Park Details Google Map Lake Winnebago -- Nagy Point boat landing, - Google Map Lake Winnebago -- Highway 45 Wayside Park Boat Ramp Details Google -

MOVEMENTS of ADULT TAGGED WALLEYES Stqcked Iri Big Lake Butte Des Morts and Spoehr;S Marsh, Wolf Uiver
.. - . Research Report No. 28 (Fisheries) MOVEMENTS OF ADULT TAGGED WALLEYES Stqcked iri Big Lake Butte des Morts and Spoehr;s Marsh, Wolf Uiver By Gordon R. Priegel DEPARTMENT OF NATURAL RESOURCES Division of Conservation Bureau of Research ca"ld Planning • INTRODUCTION This report describes the results o£ an investigation of the movements and cUspersal of walleyes captured in Rush Lake during '-Tinter and released in-Go ·v;aters coi:ltaining.an excellent natural walleye population. The waters involved in this study include Lake 1-!innebago and Big Lake Butte des Morts on the 107-mile-long Fox River and Lakes Poygan and Winneconne on the 216-mile-long Wolf River (Figure 1). The Wolf River joins the Fox River in Big Lake Butte des Morts, 10 river miles above Lake Winnebago, and then enters the lake as the Fox River at Oshkosh. The Fox River flows out of Lake Winnebago at Neenah and Menasha and flows 39 river miles north to Green Bay, Lake ~ichigan. Runoff water from 6,000 square miles enters Lake Winnebago. The lake has an area of 137,708 acres with a maximum depth of 21 feet and an average depth of 15.5 feet. It is roughly rectangular in shape: 28 miles long and 10.5 miles wide at its widest point. The small upriver lakes: Poygan, Winneconne and Big Lake Butte des Morts have areas of 14,102; 4,507 and 8,857 acres, respectively. The O.ep~chs of these smaller lakes are similar with maximum depths not exceeding ll feet. The deeper waters are located in the river channels. -

VT Baitfish Regulations Review Team Update
VT Baitfish Regulations Review Update: Comprehensive Evaluation of Fish Pathogens & Aquatic Nuisance Species (ANS) PRESENTATION TO THE VERMONT FISH & WILDLIFE BOARD FEBRUARY 21ST, 2018 TEAM MEMBERS: ADAM MILLER, SHAWN GOOD, TOM JONES, CHERYL FRANK SULLIVAN, TIM BIEBEL VT Baitfish Regulations Review Team Goal Statement: “Review the current VT baitfish regulations with the strong likelihood of coming back to the VT Fish & Wildlife Board with a revised proposal in the future to regulate baitfish use in a manner that is in the best interest of the public and protects VT’s fisheries resources.” Purpose of Today’s Presentation Review research done to date regarding evaluation of fish pathogens (including VHS) in the Northeast and surrounding area Review research done to date regarding evaluation of ANS in the Northeast and surrounding area Discuss recent enforcement action that was taken regarding a mosquitofish detection in imported fathead minnows. Gather feedback from the Board regarding research on evaluation of pathogens (including VHS) and ANS in the Northeast and surrounding area Purpose of Today’s Presentation Review research done to date regarding evaluation of fish pathogens (including VHS) in the Northeast and surrounding area Review research done to date regarding evaluation of ANS in the Northeast and surrounding area Discuss recent enforcement action that was taken regarding a mosquitofish detection in imported fathead minnows. Gather feedback from the Board regarding research on evaluation of pathogens (including VHS) and ANS in the Northeast and surrounding area Fish Pathogens of Concern Emergency Pathogens Not yet detected in Northeast. Can lead to high mortality of stock and large scale die-off of wild stocks. -

Village of Winneconne Comprehensive Outdoor
VillageVillage ofof WinneconneWinneconne ComprehensiveComprehensive OutdoorOutdoor RecreationRecreation PlanPlan 20182018 - 2022- 2022 VILLAGE OF WINNECONNE COMPREHENSIVE OUTDOOR RECREATION PLAN 2018-2022 Adopted January 9, 2018 by Park Board Adopted January 16, 2018 by Village Board Prepared by the Village Park Board, Kirk Ruetten, Director of Public Works and the East Central Wisconsin Regional Planning Commission Trish Nau, Principal Recreation Planner ACKNOWLEDGMENTS The preparation of the Village of Winneconne Comprehensive Outdoor and Recreation Plan 2018-2022 was formulated by the Winneconne Park Commission with assistance from the East Central Wisconsin Regional Planning Commission. MISSION It is the mission of the Village Parks Board to improve the quality of life for all of the Village of Winneconne’s residents and visitors by providing and promoting well-maintained parks, recreational facilities, open space, and urban forest. PARK COMMISSION The Commission is composed of seven citizen members and meets approximately once a month. The Commission works on planning park improvements and with the Director on park and recreation issues. 2017 VILLAGE BOARD John Rogers, President Andy Beiser Chris Boucher Doug Falk Ed Fischer Joe Hoenecke Jeanne Lehr PARK COMMISSION Chris Ruetten, Chair Chris Boucher Jeanne Lehr David Reetz Lani Stanek STAFF Kirk Ruetten, Director of Public Works ABSTRACT TITLE: Village of Winneconne Comprehensive Outdoor Recreation Plan 2018-2022 CONTACT: Trish Nau, ECWRPC Principal Planner AUTHORS: Village Park Board -

Lower Fox River Lakes - Fish Information
Lower Fox River Lakes - Fish Information Little Lake Butte des Morts – Fish Regulations Fish Population Season Panfish Abundant Open All Year Sturgeon Abundant Closed Catfish Abundant Open All Year Northern Pike Common Open All Year Walleye Common Open All Year Largemouth Bass Present Open All Year Smallmouth Bass Present Open All Year Lake Winnebago – Fish Regulations Fish Population Season Pan fish (bluegill, pumpkinseed, sunfish, crappie and yellow Abundant Open All Year perch) Walleye Abundant Open All Year Lake Sturgeon Abundant Closed Channel Catfish Abundant Open All Year Flathead Catfish Abundant Open All Year Largemouth Bass Common Open All Year Smallmouth Bass Common Open All Year Musky Present May -December Northern Pike Present May to March Lake Butte des Morts – Fish Regulations Fish Population Season Panfish Abundant Open All Year Northern Pike Abundant 5/14 – 3/15 Sturgeon Abundant Closed Channel Catfish Abundant Open All Year Flathead Catfish Abundant 5/14 – 9/14 Largemouth Bass Common Open All Year Musky Present 5/14 - 12/14 Smallmouth Bass Present Open All Year Walleye Present Open All Year Upper Fox River Lakes - Fish Information Lake Winneconne Fish Population Season Panfish Abundant Open All Year Walleye Common Open All Year Sturgeon Abundant Closed Channel Catfish Abundant Open All Year Largemouth Bass Common Open All Year Smallmouth Bass Present Open All Year Musky Present 5/14 - 12/14 Northern Pike Abundant 5/14 – 3/15 Lake Poygan Fish Population Season Panfish Abundant Open All Year Sturgeon Abundant Closed Channel -

Wisconsin Record Fish List Sources: the National Freshwater Fishing Hall of Fame and Verified Wisconsin Record Fish Reports Weight Length Date Species Lbs Oz
WISCONSIN DEPARTMENT OF NATURAL RESOURCES July 2019 FRESHWATER ANGLING RECORDS PO Box 7921 WISCONSIN RECORD FISH Madison, WI 53707-7921 (All Methods) Wisconsin Record Fish List Sources: The National Freshwater Fishing Hall of Fame and Verified Wisconsin Record Fish Reports Weight Length Date Species lbs oz. in. Caught by Address Caught Place Caught County KEPT HOOK AND LINE Alewife 0 2.4 8.125 Eric Geisthardt Milwaukee, WI 05/19/17 Lake Michigan Milwaukee Bass, Largemouth 11 3 none Robert Milkowski 10/12/40 Lake Ripley Jefferson Bass, Smallmouth 9 1 none Leon Stefonek 06/21/50 Indian Lake Oneida Bass, Rock 2 15 none David Harris Waupaca, WI 06/02/90 Shadow Lake Waupaca Bass, Hybrid Striped 13 14.2 28.00 Cody Schutz Marquette, WI 03/16/02 Lake Columbia Columbia Bass, Striped 1 9.3 17.0 Samuel D. Barnes Kenosha, WI 05/24/96 Fox River Kenosha Bass, White 5 3.8 22.25 Jeremy Simmons Gotham, WI 05/05/19 Mississippi River Vernon Bass, Yellow 2 12 16.1 Gary Gehrke Stoughton, WI 02/13/13 Lake Waubesa Dane Bluegill 2 9.8 12.0 Drew Garsow DePere, WI 08/02/95 Green Bay Brown Bowfin 13 1 31.6 Kevin Kelch Wausau, WI 07/19/80 Willow Flowage Oneida Buffalo, Bigmouth 76 8 49.5 Noah Labarge Ottawa, IL 06/21/13 Petenwell Flowage Adams Buffalo, Smallmouth 20 0 30.0 Mike Berg Cedar Lake, IN 12/03/99 Milwaukee River Washington Bullhead, Black 5 8 21.5 William A. Weigus Portage, IN 09/02/89 Big Falls Flowage Rusk Bullhead, Brown 4 2 17.5 Jessica Gales Eureka, WI 07/07/06 Little Green Lake Little Green Bullhead, Yellow 3 5 15.5 Isla M. -

Comprehensive Outdoor Park and Recreation Plan
2013-2017 Comprehensive Outdoor Park and Recreation Plan Winnebago County, Wisconsin Plan Assistance from ECWRPC Adopted 03/xx/2013 DRAFT Website: http://www.co.winnebago.wi.us/parks WINNEBAGO COUNTY COMPREHENSIVE OPEN SPACE AND RECREATION PLAN 2013-2017 Prepared by Winnebago County Parks Department and the East Central Wisconsin Regional Planning Commission Adopted by Committee on: xxxx, 2013 Adopted by County Board on: xxxx, 2013 i ACKNOWLEDGMENTS The Winnebago County Comprehensive Open Space and Recreation Plan was prepared with assistance from the Winnebago County Board, and the Winnebago County Outdoor Recreation Planning Committee. WINNEBAGO COUNTY BOARD OF SUPERVISORS Mark Harris, County Executive David W. Albrecht , Chairman Patrick Brennand, 1ST Vice Chair Susan T. Ermer, Clerk Nancy Barker Jeanette Diakoff Thomas Egan Paul Eisen Thomas Ellis James Englebert Chuck Farrey Jerold Finch Maribeth Gabert Ronald Grabner Jeff Hall Tim Hamblin Guy Hegg Alfred Jacobson Stan Kline Thomas Konetzke Lawrence Kriescher Kathleen Lennon Susan Locke Donald E. Miller Douglas Nelson Kenneth Neubauer Michael Norton Shiloh Ramos Marissa Reynolds Kenneth Robl Bill Roh Joanne Sievert Harold Singstock Lawrence Smith Claud Thompson Robert J. Warnke Thomas Widener Bill Wingren WINNEBAGO COUNTY PARKS AND RECREATION PLANNING COMMITTEE Jerold Finch Tom Konetzke Harold Singstock Michael Norton Donald Miller Rob Way, Parks Director Other Acknowledgements: Vicky Redlin, Winnebago County Parks Department Staff Trish Nau, Principal Recreation Planner, ECWRPC -
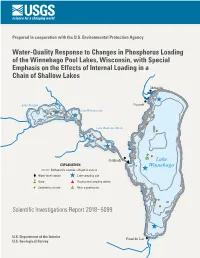
Phosphorus Inputs to the Winnebago Pool Lakes
Prepared in cooperation with the U.S. Environmental Protection Agency Water-Quality Response to Changes in Phosphorus Loading of the Winnebago Pool Lakes, Wisconsin, with Special Emphasis on the Effects of Internal Loading in a Chain of Shallow Lakes Menasha ^ Lake Poygan Neenah ! ! 2 Lake Winneconne ! ^ ! 3 1 ^ 2 ! 1 3 ! Lake Butte des Morts ! ! 7 ! ! 1 2 ^3 ! ! ! nm Oshkosh X Lake EXPLANATION Winnebago 3 Bathymetric contour—Depth in meters ^ X Water level station ^ Lake sampling site nm Buoy # Gaging and sampling station ! Sediment core site #* River sampling site 6 ! ! Scientific Investigations Report 2018–5099 5 ^ 4 1 3 U.S. Department of the Interior 2 Fond du Lac U.S. Geological Survey Cover figure. Winnebago Pool Lakes, Wisconsin, and their watersheds, with sampling locations identified. Water-Quality Response to Changes in Phosphorus Loading of the Winnebago Pool Lakes, Wisconsin, with Special Emphasis on the Effects of Internal Loading in a Chain of Shallow Lakes By Dale M. Robertson, Benjamin J. Siebers, Matthew W. Diebel, and Andrew J. Somor Prepared in cooperation with the U.S. Environmental Protection Agency Scientific Investigations Report 2018–5099 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior RYAN K. ZINKE, Secretary U.S. Geological Survey James F. Reilly II, Director U.S. Geological Survey, Reston, Virginia: 2018 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit https://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit https://store.usgs.gov. -

Winnebago County Comprehensive Outdoor Recreation Plan 2019-2023
WinnebagoTown of Dayton County ComprehensiveComprehensive OutdoorOutdoor RecreationRecreation PlanPlan 2018 - 2022 2019- 2023 * ~ East Central Wisconsin !I,! Regional Planning Commission 0 ~ ECWRPC Calumet • Fond du Lac • Menominee • Outagamie Shawano . Waupaca . Waushara . Winnebago WINNEBAGO COUNTY COMPREHENSIVE OUTDOOR RECREATION PLAN 2019-2023 Recommended March 18, 2019 by Park and Recreation Committee Adopted April 16, 2019 by County Board Prepared by the Park and Recreation Committee and the East Central Wisconsin Regional Planning Commission Trish Nau, Principal Recreation Planner iii ACKNOWLEDGMENTS The preparation of Winnebago County’s Comprehensive Outdoor and Recreation Plan 2019- 2023 was formulated by the Park and Recreation Committee with assistance from the East Central Wisconsin Regional Planning Commission. COUNTY GOVERNMENT The Park and Recreation Committee is composed of five members and meets approximately once a month. The Committee works on planning parks, recreational, and trail improvements within the County boundaries. 2018-19 WINNEBAGO COUNTY BOARD Thomas J. Konetzke, District 1 Michael A. Brunn, District 2 Thomas Borchart, District 3 Paul Eisen, District 4 Shiloh J. Ramos, District 5 Brian Defferding, District 6 Steven Lenz, District 7 Lawrence W. Smith, District 8 Timothy E. Hogan, District 9 Stephanie J. Spellman, District 10 David Albrecht, District 11 Maribeth Gabert, District 12 Steven Binder, District 13 Jesse Wallin, District 14 Vicki S. Schorse, District 15 Aaron Wojciechowski, District 16 Julie A. Gordan, District 17 Bill Wingren, District 18 Larry Lautenschlager, District 19 Michael Norton, District 20 Robert J. Warnke, District 21 Kenneth Robl, District 22 Harold Singstock, District 23 Andy Buck, District 24 Karen D. Powers, District 25 Susan Locke, District 26 Jim Wise, District 27 Jerry Finch, District 28 Rachel A. -

Trails Both Local and State in the Future
ENVIRONMENTAL ANALYSIS AND DECISION ON THE NEED FOR AN ENVIRONMENTAL IMPACT STATEMENT (EIS) Department of Natural Resources {DNA) Form 1600-8 Rev. 6·2001 Region or Bureau Bureau of Facilities and Lands Type List Designation Contact Person: NOTE TO REVIEWERS: This document is a DNA environmental analysis that evaluates probable environmental effects and decides on Richard Steffes the need for an EIS. The attached analysis includes a description of the proposal and the affected environment. The DNA has reviewed the attachments and, upon certification, accepts responsibility for their scope and content to fulfill requirements ins. NR 150.22, Wis. Adm . Code . Your comments should address completeness, accuracy or the EIS decision . For your comments to be considered, they must be received by the contact person before 4:30p.m., Insert Date. Title: Natural Resources Real Estate Director Address: Box 7921, 101 S. Webster St. Madison, WI 53707 Telephone Number 608 266 0201 E-mail Address Steffr@ dnr.state.wi.us Applicant: \VDl\'R Address: Box 7921, 101 S. Webster St; Madison, Wl53707 Title of Proposal: 2 State Trail Extensions I 4 new State Trail Projects Location: County: Forest, Langlade. Outaga mie, Kewaunee, Winnebago, Washington, Brown, Calumet, Fo nd duLac City/TownNillage: See table below and attached maps Township Range Section{s): See attached maps PROJECT SUMMARY 1. Brief overview of the proposal including the DNA action The Department has obtained an agreement to purchase approximately 1 ,500 acres of land consisting of six railroad grades from Canadian National I Illinois Central. Two grades are extensions of existing projects. These include an extension of the Ahnapee State Trail in Kewaunee County from Luxemberg to Kewaunee and an extension to the Fox River State Trail in Brown and Calumet Counties from Greenleaf to Hilbert. -

Wisconsin Trails Network Plan 2001 ACKNOWLEDGMENTS Iii
Wisconsin Trails Network Plan Open/Established Trail ○○○ Proposed Trail Wisconsin Department of Natural Resources PUB-PR-313 2003 TRAILS NETWORK PLAN TRAILS NETWORK Wisconsin Department of Natural Resources Wisconsin 2003 Trails Network Plan First Printed in January 2001 Revised in March 2003 Wisconsin Department of Natural Resources Box 7921, 101 S. Webster St. Madison, WI 53707 For more information contact the Bureau of Parks and Recreation at (608) 266-2181 The Wisconsin Department of Natural Resources provides equal opportunity in its employment, programs, services, and functions under an Affirmative Action Plan. If you have any questions, please write to Equal Opportunity Office, Department of Interior, Washington, D.C. 20240. This publication is available in alternative format (large print, Braille, audio tape, etc.) upon request. Please call the Bureau of Parks and Recreation at (608) 266-2181. ii Wisconsin Trails Network Plan 2001 ACKNOWLEDGMENTS iii George E. Meyer, Secretary State Trails Council Steve Miller, Administrator, Lands Division Connie C. Loden, Hurley, Chair Susan Black, Director, Bureau of Parks and Christopher Kegel, Mequon, Vice Chair Recreation Michael F. Sohasky, Antigo, Secretary Jeffrey L. Butson, Madison Thomas Huber, Madison 1999 Guidance Team Mike McFadzen, Plymouth Bill Pfaff, New Lisbon Dale Urso, Land Leader, Northern Region David W. Phillips, Madison ACKNOWLEDGMENTS Susan Black, Director, Bureau of Parks and Joe Parr, Brodhead Recreation Robert Roden, Director, Bureau of Lands and Facilities Others Involved -

Chironomid (Lake Fly) Relative Abundance Assessment Report
Chironomid (Lake Fly) Relative Abundance Assessment Report Ryan Koenigs, Winnebago Sturgeon and Panfish Biologist, February 21, 2018 Introduction: Chironomid larvae, commonly referred to as lake fly larvae or redworms, are very common throughout Lake Winnebago and the Upriver Lakes. In fact, Heling (2016) reported that Chironomids represented approximately half of all macroinvertebrates sampled from the profundal zone (deep zone of a body of water located below the range of effective light penetration) of Lake Winnebago in 2013 and 2014. Chironomid larvae are an important part of the aquatic food web and are critical to the diet of lake sturgeon. Stelzer et al. (2006) estimated that Chironomid larvae contribute 49% of the carbon assimilated by lake sturgeon, while gizzard shad contributed 37% comparatively. These results indicate that, although gizzard shad can dominate the diets of lake sturgeon during the winter spear fishery, Chironomid larvae are a more important food source to lake sturgeon over the course of an entire year. Periodic assessments of the Chironomid populations inhabiting Lake Winnebago were conducted between 1961-2017 (Hilsenhoff 1961, 192, 1966, 1967; Koehnke 1997; Heling 2016). Each project had slightly different objectives, but relative abundance was always assessed, thus providing a long-range data set. A standard assessment of the abundance and distribution of Chironomid larvae within the Upriver Lakes (Butte des Morts, Winneconne, and Poygan) has not been conducted to date. Our objectives for assessing the Chironomid populations on the Winnebago System are to: 1) assess relative abundance of Chironomid larvae within Lake Winnebago and the Upriver Lakes and 2) assess spatial distribution of Chironomid larvae within Lake Winnebago and the Upriver Lakes.