Variation in the Number of Testicular Follicles And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Ascalaphidae of the Afrotropical Region (Neurop Tera)
The Ascalaphidae of the Afrotropical Region (Neuroptera) 1. External morphology and bionomics of the family Ascalaphidae, and taxonomy of the subfamily Haplogleniinae including the tribes Proctolyrini n. tribe, Melambrotini n. tribe, Campylophlebini n. tribe, Tmesibasini n. tribe, Allocormodini n. tribe, and Ululomyiini n. tribe of Ascalaphinae Contents Tjeder, B. T: The Ascalaphidae of the Afrotropical Region (Neuroptera). 1. External morphology and bionomics of the family Ascalaphidae, and taxonomy of the subfamily Haplogleniinae including the tribes Proctolyrini n. tribe, Melambro- tinin. tribe, Campylophlebinin. tribe, Tmesibasini n. tribe, Allocormodini n. tribe, and Ululomyiini n. tribe of Ascalaphinae ............................................................................. 3 Tjeder, B t &Hansson,Ch.: The Ascalaphidaeof the Afrotropical Region (Neuroptera). 2. Revision of the hibe Ascalaphini (subfam. Ascalaphinae) excluding the genus Ascalaphus Fabricius ... .. .. .. .. .. .... .. .... .. .. .. .. .. .. .. .. .. 17 1 Contents Proctolyrini n. tribe ................................... .. .................60 Proctolyra n . gen .............................................................61 Introduction .........................................................................7 Key to species .............................................................62 Family Ascalaphidae Lefebvre ......................... ..... .. ..... 8 Proctolyra hessei n . sp.......................................... 63 Fossils ............................. -

Research Article Selection of Oviposition Sites by Libelloides
Hindawi Publishing Corporation Journal of Insects Volume 2014, Article ID 542489, 10 pages http://dx.doi.org/10.1155/2014/542489 Research Article Selection of Oviposition Sites by Libelloides coccajus (Denis & Schiffermüller, 1775) (Neuroptera: Ascalaphidae), North of the Alps: Implications for Nature Conservation Markus Müller,1 Jürg Schlegel,2 and Bertil O. Krüsi2 1 SKK Landschaftsarchitekten, Lindenplatz 5, 5430 Wettingen, Switzerland 2 Institute of Natural Resource Sciences, ZHAW Zurich University of Applied Sciences, Gruental,8820W¨ adenswil,¨ Switzerland Correspondence should be addressed to Markus Muller;¨ [email protected] Received 27 November 2013; Accepted 18 February 2014; Published 27 March 2014 Academic Editor: Jose´ A. Martinez-Ibarra Copyright © 2014 Markus Muller¨ et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. (1) The survival of peripheral populations is often threatened, especially in a changing environment. Furthermore, such populations frequently show adaptations to local conditions which, in turn, may enhance the ability of a species to adapt to changing environmental conditions. In conservation biology, peripheral populations are therefore of particular interest. (2) In northern Switzerland and southern Germany, Libelloides coccajus is an example of such a peripheral species. (3) Assuming that suitable oviposition sites are crucial to its long-term survival, we compared oviposition sites and adjacent control plots with regard to structure and composition of the vegetation. (4) Vegetation structure at and around oviposition sites seems to follow fairly stringent rules leading to at least two benefits for the egg clutches: (i) reduced risk of contact with adjacent plants, avoiding delayed drying after rainfall or morning dew and (ii) reduced shading and therefore higher temperatures. -

GIS-Based Modelling Reveals the Fate of Antlion Habitats in the Deliblato Sands Danijel Ivajnšič1,2 & Dušan Devetak1
www.nature.com/scientificreports OPEN GIS-based modelling reveals the fate of antlion habitats in the Deliblato Sands Danijel Ivajnšič1,2 & Dušan Devetak1 The Deliblato Sands Special Nature Reserve (DSSNR; Vojvodina, Serbia) is facing a fast successional process. Open sand steppe habitats, considered as regional biodiversity hotspots, have drastically decreased over the last 25 years. This study combines multi-temporal and –spectral remotely sensed data, in-situ sampling techniques and geospatial modelling procedures to estimate and predict the potential development of open habitats and their biota from the perspective of antlions (Neuroptera, Myrmeleontidae). It was confrmed that vegetation density increased in all parts of the study area between 1992 and 2017. Climate change, manifested in the mean annual precipitation amount, signifcantly contributes to the speed of succession that could be completed within a 50-year period. Open grassland habitats could reach an alarming fragmentation rate by 2075 (covering 50 times less area than today), according to selected global climate models and emission scenarios (RCP4.5 and RCP8.5). However, M. trigrammus could probably survive in the DSSNR until the frst half of the century, but its subsequent fate is very uncertain. The information provided in this study can serve for efective management of sand steppes, and antlions should be considered important indicators for conservation monitoring and planning. Palaearctic grasslands are among the most threatened biomes on Earth, with one of them – the sand steppe - being the most endangered1,2. In Europe, sand steppes and dry grasslands have declined drastically in quality and extent, owing to agricultural intensifcation, aforestation and abandonment3–6. -

Xavier Pons Catedràtic D'universitat
Xavier Pons Catedràtic d'Universitat Dades personals Descaregar imagen Categoria: Catedràtic d'Universitat Àrea de coneixement: Entomologia Adreça: ETSEA, Edifici Principal B, despatx 1.13.2 Telèfon: +34 973 702824 E-mail: [email protected] [ mailto:[email protected] ] Formació Acadèmica · Doctorat, Universitat Politèecnica de Catalunya (UPC), 1986 · Enginyer Agrònom, UPC, 1983 · Enginyer Tècnic en Explotacions Agropecuàries, 1978 Experiència Professional · 2002 – Actualitat: Catedràtic d’Universitat, Universitat de Lleida (UdL), Departament de Producció Vegetal i Ciència Forestal · 1996 – 2002: Professor Titular d’Universitat, UdL, Departament de Producció Vegetal i Ciència Forestal · 1986 – 1996: Profesor Titular d’Escola Universitària, UdL, Departament de Producció Vegetal i Ciència Forestal · 1982 – 1986: Profesor Associat, UPC, Escola Universitària d’Enginyeria Tècnica Agrícola de Lleida Recerca · Control integrat de plagues de cultius herbacis extensius: panís, alfals i altres. · Biologia, ecologia i control de pugons. 1 · Control integrat de plagues en espais verds urbans. Docència · INCENDIS I SANITAT FORESTAL Grau en Enginyeria Forestal · SALUT SELS BOSCOS Grau en Enginyeria Forestal · PROTECCIÓ VEGETAL Grau en Enginyeria Agrària i Alimentària · ENTOMOLOGIA AGRÍCOLA Màster Universitari en Protecció Integrada de Cultius · PROGRAMES DE PROTECCIÓ INTEGRADA DE CULTIUS Màster Universitari en Protecció Integrada de Cultius Publicacions Recents Madeira F, di Lascio, Costantini ML, Rossi L, Pons X. 2019. Intercrop movement of heteropteran predators between alfalfa and maize examined by stable isotope analysis. Jorunal of Pest Science 92: 757-76. DOI: 10.1007/s10340-018-1049-y Karp D, Chaplin-Kramer R, Meehan TD, Martin EA, DeClerck F, et al. 2018. Crop pest and predators exhibit inconsistent responses to surrounding landscape composition. -

Prey Recognition in Larvae of the Antlion Euroleon Nostras (Neuroptera, Myrrneleontidae)
Acta Zool. Fennica 209: 157-161 ISBN 95 1-9481-54-0 ISSN 0001-7299 Helsinki 6 May 1998 O Finnish Zoological and Botanical Publishing Board 1998 Prey recognition in larvae of the antlion Euroleon nostras (Neuroptera, Myrrneleontidae) Bojana Mencinger Mencinger, B., Department of Biology, University ofMaribor, Koro&a 160, SLO-2000 Maribor, Slovenia Received 14 July 1997 The behavioural responses of the antlion larva Euroleon nostras to substrate vibrational stimuli from three species of prey (Tenebrio molitor, Trachelipus sp., Pyrrhocoris apterus) were studied. The larva reacted to the prey with several behavioural patterns. The larva recognized its prey at a distance of 3 to 15 cm from the rim of the pit without seeing it, and was able to determine the target angle. The greatest distance of sand tossing was 6 cm. Responsiveness to the substrate vibration caused by the bug Pyrrhocoris apterus was very low. 1. Introduction efficient motion for antlion is to toss sand over its back (Lucas 1989). When the angle between the The larvae of the European antlion Euroleon head in resting position and the head during sand nostras are predators as well as the adults. In loose tossing is 4S0, the section of the sand tossing is substrate, such as dry sand, they construct coni- 30" (Koch 1981, Koch & Bongers 1981). cal pits. At the bottom of the pit they wait for the Sensitivity to vibration in sand has been stud- prey, which slides into the trap. Only the head ied in a few arthropods, e.g. in the nocturnal scor- and sometimes the pronotum of the larva are vis- pion Paruroctonus mesaensis and the fiddler crab ible; the other parts of the body are covered with Uca pugilator. -
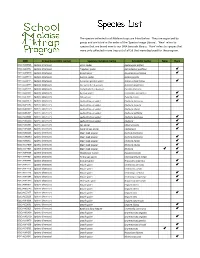
Species List
The species collected in all Malaise traps are listed below. They are organized by group and are listed in the order of the 'Species Image Library'. ‘New’ refers to species that are brand new to our DNA barcode library. 'Rare' refers to species that were only collected in one trap out of all 59 that were deployed for the program. -

From Chewing to Sucking Via Phylogeny—From Sucking to Chewing Via Ontogeny: Mouthparts of Neuroptera
Chapter 11 From Chewing to Sucking via Phylogeny—From Sucking to Chewing via Ontogeny: Mouthparts of Neuroptera Dominique Zimmermann, Susanne Randolf, and Ulrike Aspöck Abstract The Neuroptera are highly heterogeneous endopterygote insects. While their relatives Megaloptera and Raphidioptera have biting mouthparts also in their larval stage, the larvae of Neuroptera are characterized by conspicuous sucking jaws that are used to imbibe fluids, mostly the haemolymph of prey. They comprise a mandibular and a maxillary part and can be curved or straight, long or short. In the pupal stages, a transformation from the larval sucking to adult biting and chewing mouthparts takes place. The development during metamorphosis indicates that the larval maxillary stylet contains the Anlagen of different parts of the adult maxilla and that the larval mandibular stylet is a lateral outgrowth of the mandible. The mouth- parts of extant adult Neuroptera are of the biting and chewing functional type, whereas from the Mesozoic era forms with siphonate mouthparts are also known. Various food sources are used in larvae and in particular in adult Neuroptera. Morphological adaptations of the mouthparts of adult Neuroptera to the feeding on honeydew, pollen and arthropods are described in several examples. New hypoth- eses on the diet of adult Nevrorthidae and Dilaridae are presented. 11.1 Introduction The order Neuroptera, comprising about 5820 species (Oswald and Machado 2018), constitutes together with its sister group, the order Megaloptera (about 370 species), and their joint sister group Raphidioptera (about 250 species) the superorder Neuropterida. Neuroptera, formerly called Planipennia, are distributed worldwide and comprise 16 families of extremely heterogeneous insects. -

Supplementary Information
Supplementary Information A first higher-level time-calibrated phylogeny of antlions (Neuroptera: Myrmeleontidae) Bruno Michel, Anne-Laure Clamens, Olivier Béthoux, Gael J. Kergoat, Fabien L. Condamine Table S1. Taxon sampling used in this study. It contains information on the taxonomy and systematics, as well as the voucher ID, and the collection locality. It also contains the GenBank accession numbers for each molecular marker successfully sequenced. Table S2. PCR conditions (a) and PCR primers (b) used in this study to sequence the selected genes. Figure S1. The Bayesian consensus tree inferred with MrBayes on the 113-taxa and seven genes. Posterior probabilities depict node supports. Figure S2. Bayesian time-calibrated tree as inferred with BEAST (three fossil calibrations set with uniform priors, and a birth-death process a the tree prior). Figure S3. Bayesian time-calibrated tree as inferred with BEAST (four fossil calibrations set with uniform priors, and a birth-death process a the tree prior). ! ! Table S1. Taxon sampling used in this study. It contains information on the taxonomy and systematics, as well as the voucher ID, and the collection locality. It also contains the GenBank accession numbers for each molecular marker successfully sequenced. Voucher Family Subfamily Tribe Subtribe Genus Species Locality COI COIII Cytb 12S 16S 18S 28S Ascalaphidae Ascalohybris subjacens - NC_021428 NC_021428 NC_021428 NC_021428 NC_021428 KC413913 - Ascalaphidae Ascaloptynx appendiculata - NC_011277 NC_011277 NC_011277 NC_011277 NC_011277 -

Preference of Antlion and Wormlion Larvae (Neuroptera: Myrmeleontidae; Diptera: Vermileonidae) for Substrates According to Substrate Particle Sizes
Eur. J. Entomol. 112(3): 000–000, 2015 doi: 10.14411/eje.2015.052 ISSN 1210-5759 (print), 1802-8829 (online) Preference of antlion and wormlion larvae (Neuroptera: Myrmeleontidae; Diptera: Vermileonidae) for substrates according to substrate particle sizes Dušan DEVETAK 1 and AMY E. ARNETT 2 1 Department of Biology, Faculty of Natural Sciences and Mathematics, University of Maribor, Koroška cesta 160, SI-2000 Maribor, Slovenia; e-mail: [email protected] 2 Center for Biodiversity, Unity College, 90 Quaker Hill Road, Unity, ME 04915, U.S.A.; e-mail: [email protected] Key words. Neuroptera, Myrmeleontidae, Diptera, Vermileonidae, antlions, wormlions, substrate particle size, substrate selection, pit-builder, non-pit-builder, habitat selection Abstract. Sand-dwelling wormlion and antlion larvae are predators with a highly specialized hunting strategy, which either construct efficient pitfall traps or bury themselves in the sand ambushing prey on the surface. We studied the role substrate particle size plays in these specialized predators. Working with thirteen species of antlions and one species of wormlion, we quantified the substrate particle size in which the species were naturally found. Based on these particle sizes, four substrate types were established: fine substrates, fine to medium substrates, medium substrates, and coarse substrates. Larvae preferring the fine substrates were the wormlion Lampromyia and the antlion Myrmeleon hyalinus originating from desert habitats. Larvae preferring fine to medium and medium substrates belonged to antlion genera Cueta, Euroleon, Myrmeleon, Nophis and Synclisis and antlion larvae preferring coarse substrates were in the genera Distoleon and Neuroleon. In addition to analyzing naturally-occurring substrate, we hypothesized that these insect larvae will prefer the substrate type that they are found in. -

Comparative Study of Sensilla and Other Tegumentary Structures of Myrmeleontidae Larvae (Insecta, Neuroptera)
Received: 30 April 2020 Revised: 17 June 2020 Accepted: 11 July 2020 DOI: 10.1002/jmor.21240 RESEARCH ARTICLE Comparative study of sensilla and other tegumentary structures of Myrmeleontidae larvae (Insecta, Neuroptera) Fernando Acevedo Ramos1,2 | Víctor J. Monserrat1 | Atilano Contreras-Ramos2 | Sergio Pérez-González1 1Departamento de Biodiversidad, Ecología y Evolución, Unidad Docente de Zoología y Abstract Antropología Física, Facultad de Ciencias Antlion larvae have a complex tegumentary sensorial equipment. The sensilla and Biológicas, Universidad Complutense de Madrid, Madrid, Spain other kinds of larval tegumentary structures have been studied in 29 species of 2Departamento de Zoología, Instituto de 18 genera within family Myrmeleontidae, all of them with certain degree of Biología- Universidad Nacional Autónoma de psammophilous lifestyle. The adaptations for such lifestyle are probably related to México, Mexico City, Mexico the evolutionary success of this lineage within Neuroptera. We identified eight types Correspondence of sensory structures, six types of sensilla (excluding typical long bristles) and two Fernando Acevedo Ramos, Departamento de Biodiversidad, Ecología y Evolución, Unidad other specialized tegumentary structures. Both sensilla and other types of structures Docente de Zoología y Antropología Física, that have been observed using scanning electron microscopy show similar patterns in Facultad de Ciencias Biológicas, Universidad Complutense de Madrid, Madrid, Spain. terms of occurrence and density in all the studied -

Order Neuroptera, Family Ascalaphidae
Arthropod fauna of the UAE, 4: 59–65 Date of publication: 31.05.2010 Order Neuroptera, family Ascalaphidae György Sziráki INTRODUCTION Hitherto 11 species of Ascalaphidae, also known as ’owl-flies’, have been recorded from the Arabian Peninsula (Hölzel, 2004); at that moment only a single species (Ptyngidricerus venustus Tjeder & Waterston, 1977) from the United Arab Emirates was listed. Howarth and Aspinall (2002) recorded Bubopsis hamata Klug, 1834, and Gillett & Howarth (2004) listed Ascalaphus spec. from Jebel Hafit. M. Gillett & C. Gillett (2005) stated four species of Ascalaphidae were known from the UAE, but in the publication referred to by them (Gillett, 1999) only an unidentified species really from the territory of the UAE is mentioned, the others being from Oman. In the present paper five species from two subfamilies are recorded. As the sexual dimorphism, as well as the intraspecific variability may be considerable, the characteristic taxonomic features are given in a somewhat detailed form instead of a very short diagnosis. MATERIALS AND METHODS All examined specimens were collected in the UAE by Antonius van Harten, unless otherwise stated. The owl-flies were caught mainly with light traps which were operated in different parts of the country. The examined material is divided between UAE Invertebrate Collection and the collection of the Hungarian Natural History Museum. Abbreviations used in the text: AL = at light; AvH = Antonius van Harten; KS = K. Szpila; LT = light trap; MT = Malaise trap; TP = T. Pape; WT = water trap. SYSTEMATIC ACCOUNT Subfamily Haplogleniinae Newman, 1853 (Eyes entire, not devided by a horizontal sulcus.) Ptyngidricerus venustus Tjeder & Waterston, 1977 Plates 1–2 Specimens examined: Al-Ajban, 1♂, 25.ii–27.iii.2006, LT. -

Insecta : Neuroptera) 111." Distoleontini and Acanthaclisinae
Aust. J. Zool., Suppl. Ser., 1985, 106, 1-159 A Revision of the Australian Myrmeleontidae (Insecta : Neuroptera) 111." Distoleontini and Acanthaclisinae T. R. New Department of Zoology, La Trobe University, Bundoora, Vic. 3083. Abstract The Australian Myrmeleontinae : Distoleontini (64 spp.) and Acanthaclisinac (16 spp.) are revised, and keys and figures provided to enable separation of all genera and species. Two species (Distoleon nefarius Navas, Cosina vaga Navas) have not been conlirmed from Australia. New species are described of the distoleontine genera Stenogymnocnemia (one), Xantholeon (four), Stenoleon (five), Escura (six), Bandidus (of which Heteroleon Esben-Petersen is a new synonym) (22) and of the acanthaclisine genera Heoclisis (two) and Cosina (two). A new genus of Acanthaclisinae (Arcuaplectron) is also described. Introduction This final part of a revision of the Australian Myrmeleontidae includes the Myrmeleontinae : Distoleontini and the Acanthaclisinae. Both groups are well established and widely distributed in Australia and, as with other groups of ant-lions, endemicity is extremely high. Abbreviations are as used in Parts I and 11, and figure numbering continues in sequence. A check-list to all three parts is also provided. Tribe DISTOLEONTINI This tribe is well represented in Australia, and a number of genera are endemic. Many of the species are fairly 'nondescript ant-lions' and many form small groups of closely allied and generally very similar forms. Some genera are distinctive, others are not, and a world revision of this tribe is needed in order to be able to adequately assess the relationships of the Australian fauna. For some, both nomenclatorial history and taxonomic affiliation are confused.