High ABCG4 Expression Is Associated with Poor Prognosis in Non-Small-Cell Lung Cancer Patients Treated with Cisplatin-Based Chemotherapy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Design and Methods of the Prevalence and Pharmacogenomics of Tenofovir Nephrotoxicity in HIV-Positive Adults in South-Western Nigeria Study Muzamil O
Hassan et al. BMC Nephrology (2020) 21:436 https://doi.org/10.1186/s12882-020-02082-3 STUDY PROTOCOL Open Access Design and methods of the prevalence and pharmacogenomics of tenofovir nephrotoxicity in HIV-positive adults in south-western Nigeria study Muzamil O. Hassan1,2* , Raquel Duarte3, Victor O. Mabayoje4, Caroline Dickens3, Akeem O. Lasisi5 and Saraladevi Naicker6 Abstract Background: Individuals of African descent are at higher risk of developing kidney disease than their European counterparts, and HIV infection is associated with increased risk of nephropathy. Despite a safe renal profile in the clinical trials, long-term use of tenofovir disoproxil fumarate (TDF) has been associated with proximal renal tubulopathy although the underlying mechanisms remain undetermined. We aim to establish the prevalence of and risk factors for TDF-induced kidney tubular dysfunction (KTD) among HIV-I and II individuals treated with TDF in south-west Nigeria. Association between TDF-induced KTD and genetic polymorphisms in renal drug transporter genes and the APOL1 (Apolipoprotein L1) gene will be examined. Methods: This study has two phases. An initial cross-sectional study will screen 3000 individuals attending the HIV clinics in south-west Nigeria for KTD to determine the prevalence and risk factors. This will be followed by a case- control study of 400 KTD cases and 400 matched controls to evaluate single nucleotide polymorphism (SNP) associations. Data on socio-demographics, risk factors for kidney dysfunction and HIV history will be collected by questionnaire. Blood and urine samples for measurements of severity of HIV disease (CD4 count, viral load) and renal function (creatinine, eGFR, phosphate, uric acid, glucose) will also be collected. -

Genome-Wide Analysis of ATP-Binding
Tian et al. BMC Genomics (2017) 18:330 DOI 10.1186/s12864-017-3706-6 RESEARCH ARTICLE Open Access Genome-wide analysis of ATP-binding cassette (ABC) transporters in the sweetpotato whitefly, Bemisia tabaci Lixia Tian1, Tianxue Song2, Rongjun He1, Yang Zeng1, Wen Xie1, Qingjun Wu1, Shaoli Wang1, Xuguo Zhou3* and Youjun Zhang1* Abstract Background: ABC transporter superfamily is one of the largest and ubiquitous groups of proteins. Because of their role in detoxification, insect ABC transporters have gained more attention in recent years. In this study, we annotated ABC transporters from a newly sequenced sweetpotato whitefly genome. Bemisia tabaci Q biotype is an emerging global invasive species that has caused extensive damages to field crops as well as ornamental plants. Results: A total of 55 ABC transporters containing all eight described subfamilies (A to H) were identified in the B. tabaci Q genome, including 8 ABCAs, 3 ABCBs, 6 ABCCs, 2 ABCDs, 1 ABCE, 3 ABCFs, 23 ABCGs and 9 ABCHs. In comparison to other species, subfamilies G and H in both phloem- and blood-sucking arthropods are expanded. The temporal expression profiles of these 55 ABC transporters throughout B. tabaci developmental stages and their responses to imidacloprid, a neonicotinoid insecticide, were investigated using RNA-seq analysis. Furthermore, the mRNA expression of 24 ABC transporters (44% of the total) representing all eight subfamilies was confirmed by the quantitative real-time PCR (RT-qPCR). Furthermore, mRNA expression levels estimated by RT-qPCR and RNA-seq analyses were significantly correlated (r =0.684,p <0.01). Conclusions: It is the first genome-wide analysis of the entire repertoire of ABC transporters in B. -

Evolutionary and Functional Studies on Microsporidian ATP-Binding
Infection, Genetics and Evolution 68 (2019) 136–144 Contents lists available at ScienceDirect Infection, Genetics and Evolution journal homepage: www.elsevier.com/locate/meegid Evolutionary and functional studies on microsporidian ATP-binding T cassettes: Insights into the adaptation of microsporidia to obligated intracellular parasitism Qiang Hea, Charles R. Vossbrinckc, Qiong Yangd, Xian-Zhi Menga, Jian Luoa, Guo-Qing Pana, ⁎ ⁎ Ze-Yang Zhoua,b, , Tian Lia, a State Key Laboratory of Silkworm Genome Biology, Southwest University, Chongqing 400715, China b College of Life Science, Chongqing Normal University, Chongqing 400047, China c Department of Soil and Water, The Connecticut Agricultural Experiment Station, 123 Huntington Street, New Haven, CT 06511, USA d Sericulture and Agri-Food Research Institute, Guangdong Academy of Agricultural Sciences, Guangzhou, China ARTICLE INFO ABSTRACT Keywords: ATP-binding cassette (ABC) transporters comprise the largest family of transmembrane proteins and are found in Microsporidian all domains of life. The ABCs are involved in a variety of biological processes and as exporters play important ABC transporter roles in multidrug resistance. However, the ABC transporters have not been addressed in microsporidia, which Genome are a very large group of obligate intracellular parasites that can infect nearly all animals, including humans. Evolution Here, a total of 234 ABC transporters were identified from 18 microsporidian genomes and classified intofive Nosema bombycis subfamilies, including 74 ABCBs, 2 ABCCs, 18 ABCEs, 15 ABCFs, 102 ABCGs and 23 uncategorized members. Two subfamilies, ABCA and ABCD, are found in most organisms, but lost in microsporidia. Phylogenetic analysis indicated that microsporidian ABCB and ABCG subfamilies expanded by recent gene duplications, which re- sulted in the two largest subfamilies in microsporidia. -

Polymorphisms of the Multidrug Pump ABCG2: a Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics
1521-009X/46/12/1886–1899$35.00 https://doi.org/10.1124/dmd.118.083030 DRUG METABOLISM AND DISPOSITION Drug Metab Dispos 46:1886–1899, December 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Minireview Polymorphisms of the Multidrug Pump ABCG2: A Systematic Review of Their Effect on Protein Expression, Function, and Drug Pharmacokinetics Niall Heyes, Parth Kapoor, and Ian D. Kerr School of Life Sciences, Queen’s Medical Centre, University of Nottingham, Nottingham, United Kingdom Received June 12, 2018; accepted September 20, 2018 Downloaded from ABSTRACT The widespread expression and polyspecificity of the multidrug to improve outcomes in cancer patients treated with tyrosine ABCG2 efflux transporter make it an important determinant of the kinase inhibitors, but the reasons for this are yet to be estab- pharmacokinetics of a variety of substrate drugs. Null ABCG2 lished, and this residue’s role in the mechanism of the protein is expression has been linked to the Junior blood group. Polymor- unexplored by current biochemical and structural approaches. phisms affecting the expression or function of ABCG2 may have Research into the less-common polymorphisms is confined to dmd.aspetjournals.org clinically important roles in drug disposition and efficacy. The in vitro studies, with several polymorphisms shown to decrease most well-studied single nucleotide polymorphism (SNP), Q141K resistance to anticancer agents such as SN-38 and mitoxantrone. (421C>A), is shown to decrease ABCG2 expression and activity, In this review, we present a systematic analysis of the effects of resulting in increased total drug exposure and decreased re- ABCG2 polymorphisms on ABCG2 function and drug pharmaco- sistance to various substrates. -

Identification of Candidate ATP-Binding Cassette Transporter
www.nature.com/scientificreports OPEN Identifcation of candidate ATP- binding cassette transporter gene family members in Diaphorina citri (Hemiptera: Psyllidae) via adult tissues transcriptome analysis Zhengbing Wang 1, Fajun Tian1, Lijun Cai2, Jie Zhang2, Jiali Liu1 & Xinnian Zeng1* The ATP-binding cassette (ABC) transporters exist in all living organisms and play major roles in various biological functions by transporting a wide variety of substrates across membranes. The functions of ABC transporters in drug resistance have been extensively studied in vertebrates; however, they are rarely characterized in agricultural pests. The Asian citrus psyllid, Diaphorina citri, is one of the most damaging pests of the Citrus genus because of its transmission of Huanglongbing, also known as Yellow Dragon disease. In this study, the next-generation sequencing technique was applied to research the ABC transporters of D. citri. Fifty-three ABC transporter genes were found in the RNA-Seq data, and among these ABC transporters, 4, 4, 5, 2, 1, 4, 18 and 15 ABC proteins belonged to the ABCA-ABCH subfamilies, respectively. Diferent expression profles of 52 genes between imidacloprid-resistant and imidacloprid-susceptible strains were studied by qRT-PCR; 5 ABCGs and 4 ABCHs were signifcantly upregulated in the imidacloprid-resistant strain. In addition, fve of the nine upregulated genes were widely expressed in adult tissues in spatial expression analysis. The results suggest that these genes may play key roles in this phenotype. In general, this study contributed to our current understanding of D. citri resistance to insecticides. Te ATP-binding cassette (ABC) transporter family is one of the largest families of membrane proteins and uni- versally exists in all living organisms on Earth1. -

Developmental Differences in the Expression of ABC Transporters at Rat Brain Barrier Interfaces Following Chronic Exposure to Di
www.nature.com/scientificreports OPEN Developmental diferences in the expression of ABC transporters at rat brain barrier interfaces Received: 28 November 2018 Accepted: 28 March 2019 following chronic exposure to Published: xx xx xxxx diallyl sulfde Liam M. Koehn1, Katarzyna M. Dziegielewska1, Kjeld Møllgård 2, Elodie Saudrais3, Nathalie Strazielle3,4, Jean-Francois Ghersi-Egea3, Norman R. Saunders 1 & Mark D. Habgood1 Many pregnant women and prematurely born infants require medication for clinical conditions including cancer, cardiac defects and psychiatric disorders. In adults drug transfer from blood into brain is mostly restricted by efux mechanisms (ATP-binding cassette, ABC transporters). These mechanisms have been little studied during brain development. Here expression of eight ABC transporters (abcb1a, abcb1b, abcg2, abcc1, abcc2, abcc3, abcc4, abcc5) and activity of conjugating enzyme glutathione-s- transferase (GST) were measured in livers, brain cortices (blood-brain-barrier) and choroid plexuses (blood-cerebrospinal fuid, CSF, barrier) during postnatal rat development. Controls were compared to animals chronically injected (4 days, 200 mg/kg/day) with known abcb1a inducer diallyl sulfde (DAS). Results reveal both tissue- and age-dependent regulation. In liver abcb1a and abcc3 were up- regulated at all ages. In cortex abcb1a/b, abcg2 and abcc4/abcc5 were up-regulated in adults only, while in choroid plexus abcb1a and abcc2 were up-regulated only at P14. DAS treatment increased GST activity in livers, but not in cortex or choroid plexuses. Immunocytochemistry of ABC transporters at the CSF-brain interface showed that PGP and BCRP predominated in neuroepithelium while MRP2/4/5 were prominent in adult ependyma. These results indicate an age-related capacity of brain barriers to dynamically regulate their defence mechanisms when chronically challenged by xenobiotic compounds. -

ABCG4: a Novel Human White Family ABC-Transporter Expressed in the Brain and Eye
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1591 (2002) 175–179 www.bba-direct.com Short sequence-paper ABCG4: a novel human white family ABC-transporter expressed in the brain and eye Susan Oldfield *, Christopher A. Lowry, Jon Ruddick, Stafford L. Lightman University Research Centre for Neuroendocrinology, BRI, University of Bristol, Marlborough Street, Bristol BS2 8HW, UK Received 24 July 2001; received in revised form 11 April 2002; accepted 24 May 2002 Abstract White family transporters are a group of ATP-binding cassette (ABC) proteins that show sequence homology to the Drosophila white gene product. The Drosophila protein is a subunit of heterodimeric transporters of precursors for eye-pigment synthesis. A novel, human member of this family (ABCG4) has been identified. Northern blotting shows that ABCG4 is expressed specifically in the brain and the eye. D 2002 Elsevier Science B.V. All rights reserved. Keywords: White gene; ABC-transporter; ABCG1; Sterol ATP-binding cassette (ABC) transporters form a large and its murine counterpart is known as ABCG1 or ABC8. superfamily of proteins that mediate the transport of a large Northern blotting has shown that the mRNAs encoding variety of diverse molecules across biological membranes in ABCG1 are present in several tissues, predominantly brain, an ATP-dependent manner [1]. The functional transporters spleen and lung [10]. contain two ATP-binding folds and two transmembrane The function of ABCG1 is unknown, although there is domains each with, usually, five or six membrane spanning evidence that suggests that it may have a role in cholesterol helices. -

Bile Acid Transporters and Regulatory Nuclear Receptors in the Liver and Beyond
Review Bile acid transporters and regulatory nuclear receptors in the liver and beyond ⇑ Emina Halilbasic, Thierry Claudel, Michael Trauner Hans Popper Laboratory of Molecular Hepatology, Division of Gastroenterology and Hepatology, Department of Internal Medicine III, Medical University of Vienna, Austria Summary logical functions including stimulation of bile flow, intestinal absorption of lipophilic nutrients, solubilization and excretion of Bile acid (BA) transporters are critical for maintenance of the enter- cholesterol, as well as antimicrobial and metabolic effects. Tight ohepatic BA circulation where BAs exert their multiple physio- regulation of BA transporters via nuclear receptors is necessary to maintain proper BA homeostasis. Hereditary and acquired defects of BA transporters are involved in the pathogenesis of sev- Keywords: Bile acids, Cholestasis; Fatty liver disease; Gallstones; Liver regener- eral hepatobiliary disorders including cholestasis, gallstones, fatty ation; Liver cancer. liver disease and liver cancer, but also play a role in intestinal and Received 12 January 2012; received in revised form 1 August 2012; accepted 3 August metabolic disorders beyond the liver. Thus, pharmacological mod- 2012 ification of BA transporters and their regulatory nuclear receptors ⇑ Corresponding author. Address: Hans Popper Laboratory of Molecular Hepa- tology, Division of Gastroenterology and Hepatology, Department of Internal opens novel treatment strategies for a wide range of disorders. Medicine III, Medical University of Vienna, Waehringer Waehringer Guertel 18- Ó 2012 European Association for the Study of the Liver. Published 20, A-1090 Vienna, Austria. Tel.: +43 01 40400 4741; fax: +43 01 40400 4735. by Elsevier B.V. All rights reserved. E-mail address: [email protected] (M. Trauner). -

Transcriptional and Post-Transcriptional Regulation of ATP-Binding Cassette Transporter Expression
Transcriptional and Post-transcriptional Regulation of ATP-binding Cassette Transporter Expression by Aparna Chhibber DISSERTATION Submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in Pharmaceutical Sciences and Pbarmacogenomies in the Copyright 2014 by Aparna Chhibber ii Acknowledgements First and foremost, I would like to thank my advisor, Dr. Deanna Kroetz. More than just a research advisor, Deanna has clearly made it a priority to guide her students to become better scientists, and I am grateful for the countless hours she has spent editing papers, developing presentations, discussing research, and so much more. I would not have made it this far without her support and guidance. My thesis committee has provided valuable advice through the years. Dr. Nadav Ahituv in particular has been a source of support from my first year in the graduate program as my academic advisor, qualifying exam committee chair, and finally thesis committee member. Dr. Kathy Giacomini graciously stepped in as a member of my thesis committee in my 3rd year, and Dr. Steven Brenner provided valuable input as thesis committee member in my 2nd year. My labmates over the past five years have been incredible colleagues and friends. Dr. Svetlana Markova first welcomed me into the lab and taught me numerous laboratory techniques, and has always been willing to act as a sounding board. Michael Martin has been my partner-in-crime in the lab from the beginning, and has made my days in lab fly by. Dr. Yingmei Lui has made the lab run smoothly, and has always been willing to jump in to help me at a moment’s notice. -
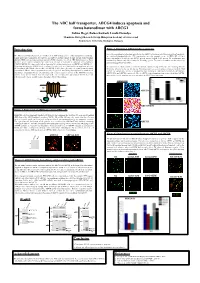
The ABC Half Transporter, ABCG4 Induces Apoptosis and Forms Heterodimer with ABCG1
The ABC half transporter, ABCG4 induces apoptosis and forms heterodimer with ABCG1 Zoltán Hegyi, Balázs Sarkadi, László Homolya Membrane Biology Research Group, Hungarian Academy of Sciences and Semmelweis University, Budapest, Hungary Introduction Figure 3. Functional ABCG4 induces apoptosis Since the morphological alterations observed in the ABCG4-expressing cells were indicative of apoptosis, The ABCG1 and ABCG4 proteins are members of the ATP binding cassette (ABC) transporter G subfamily. we examined phosphatidyl-serine (PS) translocation, an early apoptotic event in HEK293H cell lines Unlike most ABC transporters, the ABCG1 and ABCG4 proteins consist of only one nucleotide binding transiently transfected with the wt ABCG4 and its inactive mutant (KM) variant. PS translocation was domain (NBD) and one transmembrane domain (TMD), therefore are called ABC half-transporters. Some monitored by fluorescently labeled Annexin V binding (green). The total cell number was determined by members of the ABCG subfamily have proven to function as homodimers (ABCG2) or heterodimers nuclear staining by Hoechst (blue). (ABCG5/ABCG8). Previous results indicated potential heterodimerization between ABCG1 and ABCG4 (1). Regarding their function, ABCG1 has been proposed to play a role in cellular lipid/sterol regulation, whereas We found that the cultures transfected with wt ABCG4 contained a large number of cells exhibiting Annexin the function of ABCG4, the closest relative of ABCG1, is still elusive. Recently, we reported that functional V binding, whereas hardly any labeling for PS translocation was seen in cultures transfected with the KM expression of ABCG1 induces apoptosis in several cell types (2). Our finding was supported by rounded cell mutant. For comparison, Annexin V binding was also examined in cells transfected with the wt ABCG1, morphology, phosphatidyl-serine externalization, and elevated caspase 3 activity in the ABCG1-expressing ABCG1-KM, and ABCG2, respectively. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Computational Modeling to Predict the Functions and Impact of Drug Transporters Pär Matsson1,2* and Christel a S Bergström1,2*
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Matsson and Bergström In Silico Pharmacology (2015) 3:8 DOI 10.1186/s40203-015-0012-3 REVIEW Open Access Computational modeling to predict the functions and impact of drug transporters Pär Matsson1,2* and Christel A S Bergström1,2* Abstract Transport proteins are important mediators of cellular drug influx and efflux and play crucial roles in drug distribution, disposition and clearance. Drug-drug interactions have increasingly been found to occur at the transporter level and, hence, computational tools for studying drug-transporter interactions have gained in interest. In this short review, we present the most important transport proteins for drug influx and efflux. Computational tools for predicting and understanding the substrate and inhibitor interactions with these membrane-bound proteins are discussed. We have primarily focused on ligand-based and structure-based modeling, for which the state-of-the-art and future challenges are also discussed. Keywords: Drug transport; Membrane transporter; Carrier-mediated transport; Structure-activity relationship; Ligand-based modeling; Structure-based modeling Introduction Export Pump (BSEP; ABCB11), and the members of the Transport proteins, which are expressed in all tissues of multidrug resistance-associated protein family (MRP; the body, facilitate the transmembrane transport of essen- ABCC) (Giacomini et al. 2010, Hillgren et al. 2013). tial solutes such as nutrients and signal substances. They About 40 human ABC transporters are known to also play an important role in the removal of metabolites date, while more than 350 SLC transporters have been and toxicants from cells and tissues.