Nagelkerken Et Al, 2000.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potential of Pigeon Creek As a Nursery Habitat for Juvenile Reef Fish
ORIGINAL ARTICLE Potential of Pigeon Creek, San Salvador, Bahamas, As Nursery Habitat for Juvenile Reef Fish Ian C. Conboy1 Northeast Fisheries Science Center, Woods Hole, MA James M. Haynes The College at Brockport, State University of New York, Brockport, NY ABSTRACT This project assessed the significance of Pigeon Creek, San Salvador, Bahamas as a nursery habitat for coral reef fishes. Pigeon Creek’s perimeter is lined with mangrove and limestone bedrock. The bottom is sand or sea grass and ranges in depth from exposed at low tide to a 3-m deep, tide-scoured channel. In June 2006 and January 2007, fish were counted and their maturity was recorded while sampling 112 of 309 possible 50-m transects along the perimeter of the Pigeon Creek. Excluding silversides (Atherinidae, 52% of fish counted), six families each comprised more than 1% of the total abundance (Scaridae/parrotfishes, 35.3%; Lutjanidae/snappers, 23.9%; Haemulidae/grunts, 21.0%; Gerreidae/mojarras, 8.5%; Pomacentridae/damselfishes, 6.1%; Labridae/wrasses, 2.4%). There were few differences in effort-adjusted counts among habitats (mangrove, bedrock, mixed), sections (north, middle, southwest) and seasons (summer 2006 and winter 2007). Red mangrove (Rhizophora mangle), covering 68% of the perimeter was where 62% of the fish were counted. Snappers, grunts and parrot fishes are important food fishes and significant families in terms of reef ecology around San Salvador. Mangrove was the most important habitat for snappers and grunts; bedrock was most important for parrot fishes. The southwest section was important for snappers, grunts and parrot fishes, the north section for grunts and parrot fishes, and the middle section for snappers. -

Life History Demographic Parameter Synthesis for Exploited Florida and Caribbean Coral Reef Fishes
Please do not remove this page Life history demographic parameter synthesis for exploited Florida and Caribbean coral reef fishes Stevens, Molly H; Smith, Steven Glen; Ault, Jerald Stephen https://scholarship.miami.edu/discovery/delivery/01UOML_INST:ResearchRepository/12378179400002976?l#13378179390002976 Stevens, M. H., Smith, S. G., & Ault, J. S. (2019). Life history demographic parameter synthesis for exploited Florida and Caribbean coral reef fishes. Fish and Fisheries (Oxford, England), 20(6), 1196–1217. https://doi.org/10.1111/faf.12405 Published Version: https://doi.org/10.1111/faf.12405 Downloaded On 2021/09/28 21:22:59 -0400 Please do not remove this page Received: 11 April 2019 | Revised: 31 July 2019 | Accepted: 14 August 2019 DOI: 10.1111/faf.12405 ORIGINAL ARTICLE Life history demographic parameter synthesis for exploited Florida and Caribbean coral reef fishes Molly H. Stevens | Steven G. Smith | Jerald S. Ault Rosenstiel School of Marine and Atmospheric Science, University of Miami, Abstract Miami, FL, USA Age‐ or length‐structured stock assessments require reliable life history demo‐ Correspondence graphic parameters (growth, mortality, reproduction) to model population dynamics, Molly H. Stevens, Rosenstiel School of potential yields and stock sustainability. This study synthesized life history informa‐ Marine and Atmospheric Science, University of Miami, 4600 Rickenbacker Causeway, tion for 84 commercially exploited tropical reef fish species from Florida and the Miami, FL 33149, USA. U.S. Caribbean (Puerto Rico and the U.S. Virgin Islands). We attempted to identify a Email: [email protected] useable set of life history parameters for each species that included lifespan, length Funding information at age, weight at length and maturity at length. -

MRAG South Atlantic PSA Draft Report
South Atlantic Species Productivity – Susceptibility Analyses Draft Report To the Lenfest Ocean Program MRAG Americas 65 Eastern Avenue, Unit B2C Essex, MA 01929 Ph. 978-768-3880 Fax. 978-768-3878 www.mragamericas.com August 27, 2008 Table of Contents 1 Introduction........................................................................................................................................... 1 1.1 The Risk Based Assessment ........................................................................................................... 1 1.2 Information Collection ...................................................................................................................... 3 1.3 A Note about our Productivity Susceptibility Analysis Methodology................................................ 3 2 Non Snapper/Grouper Species ............................................................................................................ 3 2.1 Pink Shrimp, Penaeus [Farfantepenaeus] duorarum ....................................................................... 3 2.2 Red Drum, Sciaenops ocellatus ....................................................................................................... 4 3 Snapper/Grouper Complex .................................................................................................................. 5 3.1 Groupers .......................................................................................................................................... 5 3.2 Snapper......................................................................................................................................... -

Saltwater Fish Identification Guide
Identification Guide To South Carolina Fishes Inshore Fishes Red Drum (Spottail, redfish, channel bass, puppy drum,) Sciaenops ocellatus May have multiple spots along dorsal surface.. RKW Black Drum Pogonias cromis Broad black vertical bars along body. Barbells on chin. Spotted Seatrout (Winter trout, speckled trout) Cynoscion nebulosus Numerous distinct black spots on dorsal surface. Most commonly encountered in rivers and estuaries. RKW Most commonly encountered just offshore around live bottom and artificial reefs. Weakfish (Summer trout, Gray trout) Cynoscion regalis RKW Silver coloration with no spots. Large eye Silver Seatrout Cynoscion nothus RKW Spot Leiostomus xanthurus Distinct spot on shoulder. RKW Atlantic Croaker (Hardhead) Micropogonias undulatus RKW Silver Perch (Virginia Perch) Bairdiella chrysoura RKW Sheepshead Archosargus probatocephalus Broad black vertical bars along body. RKW Pinfish (Sailors Choice) Lagodon rhomboides Distinct spot. RKW Southern Kingfish (Whiting) Menticirrhus americanus RKW Extended 1st dorsal filament Northern Kingfish SEAMAP- Menticirrhus saxatilis SA:RPW Dusky 1st dorsal-fin tip Black caudal fin tip Gulf Kingfish SEAMAP- Menticirrhus littoralis SA:RPW Southern flounder Paralichthys lethostigma No ocellated spots . RKW Summer flounder Paralichthys dentatus Five ocellated spots in this distinct pattern. B. Floyd Gulf flounder Paralichthys albigutta B. Floyd Three ocellated spots in a triangle pattern. B. Floyd Bluefish Pomatomus saltatrix RKW Inshore Lizardfish Synodus foetens RKW RKW Ladyfish Elops saurus Florida Pompano Trachinotus carolinus RKW Lookdown Selene vomer RKW Spadefish Chaetodipterus faber Juvenile RKW Juvenile spadefish are commonly found in SC estuaries. Adults, which look very similar to the specimen shown above, are common inhabitants of offshore reefs. Cobia Rachycentron canadum Adult D. Hammond Juvenile RKW D. -

Andrew David Dorka Cobián Rojas Felicia Drummond Alain García Rodríguez
CUBA’S MESOPHOTIC CORAL REEFS Fish Photo Identification Guide ANDREW DAVID DORKA COBIÁN ROJAS FELICIA DRUMMOND ALAIN GARCÍA RODRÍGUEZ Edited by: John K. Reed Stephanie Farrington CUBA’S MESOPHOTIC CORAL REEFS Fish Photo Identification Guide ANDREW DAVID DORKA COBIÁN ROJAS FELICIA DRUMMOND ALAIN GARCÍA RODRÍGUEZ Edited by: John K. Reed Stephanie Farrington ACKNOWLEDGMENTS This research was supported by the NOAA Office of Ocean Exploration and Research under award number NA14OAR4320260 to the Cooperative Institute for Ocean Exploration, Research and Technology (CIOERT) at Harbor Branch Oceanographic Institute-Florida Atlantic University (HBOI-FAU), and by the NOAA Pacific Marine Environmental Laboratory under award number NA150AR4320064 to the Cooperative Institute for Marine and Atmospheric Studies (CIMAS) at the University of Miami. This expedition was conducted in support of the Joint Statement between the United States of America and the Republic of Cuba on Cooperation on Environmental Protection (November 24, 2015) and the Memorandum of Understanding between the United States National Oceanic and Atmospheric Administration, the U.S. National Park Service, and Cuba’s National Center for Protected Areas. We give special thanks to Carlos Díaz Maza (Director of the National Center of Protected Areas) and Ulises Fernández Gomez (International Relations Officer, Ministry of Science, Technology and Environment; CITMA) for assistance in securing the necessary permits to conduct the expedition and for their tremendous hospitality and logistical support in Cuba. We thank the Captain and crew of the University of Miami R/V F.G. Walton Smith and ROV operators Lance Horn and Jason White, University of North Carolina at Wilmington (UNCW-CIOERT), Undersea Vehicle Program for their excellent work at sea during the expedition. -

Snapper Grouper Complex
4.1.2 Snapper Grouper Complex 4.1.2.1 Species Descriptions Sea basses and Groupers (Serranidae) (all species’ descriptions updated from the Snapper Grouper SAFE Report Nov. 2005) All serranids described in this document are reported to be protogynous hermaphrodites meaning that all individuals change sex from female to male at a certain size and/or age. This size and age at sex transition is species-dependent and can vary considerably within species. Gag Gag, Mycteroperca microlepis, occur in the Western Atlantic from North Carolina to the Yucatan Peninsula, and throughout the Gulf of Mexico. Juveniles are sometimes observed as far north as Massachusetts (Heemstra and Randall 1993). Gag commonly occurs at depths of 39- 152 m (131-498 ft) (Heemstra and Randall 1993) and prefers inshore-reef and shelf-break habitats (Hood and Schlieder 1992). Bullock and Smith (1991) indicated that gag probably do not move seasonally between reefs in the Gulf of Mexico, but show a gradual shift toward deeper water with age. McGovern et al. (2005) reported extensive movement of gag along the Southeast United States. In a tagging study, 23% of the 435 recaptured gag moved distances greater that 185 km. Most of these individuals were tagged off South Carolina and were recaptured off Georgia, Florida, and in the Gulf of Mexico (McGovern et al. 2005). Gag are considered estuarine dependent (Keener et al. 1988; Ross and Moser 1995; Koenig and Coleman 1998; Strelcheck et al. 2003). Juveniles (age 0) occur in shallow grass beds along Florida’s east coast during the late spring and summer (Bullock and Smith 1991). -

University of Miami US Department of Commerce Miami-Dade County
Fisheries assessment of Biscayne Bay 1983 Item Type monograph Authors Berkeley, Steven A. Publisher NOAA/National Ocean Service Download date 01/10/2021 13:52:32 Link to Item http://hdl.handle.net/1834/30510 NOAA/University of Miami Joint Publication NOAA Technical Memorandum NOS NCCOS CCMA 166 University of Miami RSMAS TR 2004-01 Coastal and Estuarine Data Archaeology and Rescue Program University of Miami Rosenstiel School of Marine and Atmospheric Science February 2004 Miami, FL US Department of Commerce Miami-Dade County National Oceanic and Atmospheric Department of Environmental Administration Resources Management Silver Spring, MD Miami, FL a NOAA/University of Miami Joint Publication NOAA Technical Memorandum NOS NCCOS CCMA 166 University of Miami RSMAS TR TR 2004-01 Fisheries Assessment of Biscayne Bay 1983 Steven A. Berkeley Rosenstiel School of Marine and Atmospheric Science University of Miami Prepared for: Metropolitan Dade County Department of Environmental Resources Management A. Y. Cantillo NOAA National Ocean Service (Editor, 2004) February 2004 United States National Oceanic and Department of Commerce Atmospheric Administration National Ocean Service Donald L. Evans Conrad C. Lautenbacher, Jr. Jamison S. Hawkins Secretary Vice-Admiral (Ret.), Acting Assistant Administrator Administrator For further information please call or write: NOAA National Ocean Service National Centers for Coastal Ocean Science 1305 East West Hwy. Silver Spring, MD 20910 301 713 3020 COVER PHOTO: Pat Cope (Rosenstiel School of Marine and Atmospheric Science) interviewing a fisherman on the causeway leading to Miami Beach during the fisheries assessment. Photograph taken by Stephen Carney while at the Rosenstiel School of Marine and Atmospheric Science, University of Miami. -

Nbr First Edition Update Table 4.2 Southeast Region Fish
NBR FIRST EDITION UPDATE TABLE 4.2 SOUTHEAST REGION FISH BYCATCH BY STOCKS AND SPECIES - 2010 The Southeast Region generated 2010 fish bycatch estimates and ratios using 2006- 2010 data, except where otherwise noted. Species bycatch ratio = total regional bycatch of a species / (total regional landings of the species + total regional bycatch of the species). Landings data were not available at the stock level. Some bycatch ratios (marked **) could not be developed, e.g., where bycatch was by weight and numbers of individuals, and landings were in pounds. Bycatch ratio cells are left blank when landings = 0. BYCATCH SPECIES BYCATCH BYCATCH LANDINGS BYCATCH COMMON NAME SCIENTIFIC NAME (POUNDS) (INDIVIDUALS) (POUNDS) RATIO FOOTNOTE(S) ACANTHURIDAE (group) Acanthuridae (group) 77.27 Acanthuridae 0 ACANTHURIDAE (group) (Subtotal) 0.00 77.27 AFRICAN POMPANO African pompano 121.89 Alectis ciliaris 9,321 ** AFRICAN POMPANO (Subtotal) 0.00 121.89 ALBACORE Albacore 1,918.02 214.41 Thunnus alalunga 135,931 ** ALBACORE (Subtotal) 1,918.02 214.41 ALMACO JACK Almaco jack 1,680.06 Seriola rivoliana 244,114 ** ALMACO JACK (Subtotal) 0.00 1,680.06 AMBERFISHES, BANDED RUDDERFISH, AMBERJACKS, AND YELLOWTAILS (group) Amberfishes, banded rudderfish, amberjacks, and 137,243.72 yellowtails (group) Seriola 0 AMBERFISHES, BANDED RUDDERFISH, AMBERJACKS, AND 0.00 137,243.72 YELLOWTAILS (group) (Subtotal) ATLANTIC ANGELSHARK Atlantic angelshark 119.89 Squatina dumeril 0 ATLANTIC ANGELSHARK (Subtotal) 0.00 119.89 ATLANTIC BONITO Atlantic bonito 21,317.97 Sarda sarda -

Baseline Multispecies Coral Reef Fish Stock Assessment for the Dry Tortugas
NOAA Technical Memorandum NMFS-SEFSC-487 Baseline Multispecies Coral Reef Fish Stock Assessment for the Dry Tortugas Jerald S. Ault, Steven G. Smith, Geoffrey A. Meester, Jiangang Luo, James A. Bohnsack, and Steven L. Miller U.S. Department of Commerce National Oceanic and Atmospheric Administration National Marine Fisheries Service Southeast Fisheries Science Center 75 Virginia Beach Drive Miami, Florida 33149 August 2002 NOAA Technical Memorandum NMFS-SEFSC-487 Baseline Multispecies Coral Reef Fish Stock Assessment for the Dry Tortugas Jerald S. Ault 1, Steven G. Smith 1, Geoffrey A. Meester 1, Jiangang Luo 1, James A. Bohnsack 2 , and Steven L. Miller3 with significant contributions by Douglas E. Harper2, Dione W. Swanson3, Mark Chiappone3, Erik C. Franklin1, David B. McClellan2, Peter Fischel2, and Thomas W. Schmidt4 _____________________________ U.S. DEPARTMENT OF COMMERCE Donald L. Evans, Secretary National Oceanic and Atmospheric Administration Conrad C. Lautenbacher, Jr., Under Secretary for Oceans and Atmosphere National Marine Fisheries Service William T. Hogarth, Assistant Administrator for Fisheries August 2002 This technical memorandum series is used for documentation and timely communication of preliminary results, interim reports, or special purpose information. Although the memoranda are not subject to complete formal review, editorial control, or detailed editing, they are expected to reflect sound professional work. 1 University of Miami, Rosenstiel School of Marine and Atmospheric Sciences, Miami, FL 2 NOAA/Fisheries Southeast Fisheries Science Center, Miami, FL 3 National Undersea Research Center, Key Largo, FL 4 National Park Service, Homestead, FL NOTICE The National Marine Fisheries Service (NMFS) does not approve, recommend, or endorse any proprietary product or material mentioned in this publication. -

Blue Striped Grunt) Family: Haemulidae (Grunts) Order: Perciformes (Perch and Allied Fish) Class: Actinopterygii (Ray-Finned Fish)
UWI The Online Guide to the Animals of Trinidad and Tobago Diversity Haemulon sciurus (Blue Striped Grunt) Family: Haemulidae (Grunts) Order: Perciformes (Perch and Allied Fish) Class: Actinopterygii (Ray-finned Fish) Fig. 1. Blue striped grunt, Haemulon sciurus. [http://www.reefcolors.com/Galleries/2009_MariaLaGorda_IIb/photos/TG_238_6.jpg, downloaded 17 October 2016] TRAITS. The blue striped grunt is a dusky yellow almond-shaped fish with blue horizontal stripes (Fig. 1). The upper jaw of these fish reaches below the centre of the eye, and they have oblique scales below the lateral line and enlarged ones above the lateral line (Bester, 2016). The general adult length ranges from 36-46cm and the maximum recorded weight of a blue striped grunt is 0.75kg (Froese and Pauly, 2013). Young ones have a rapid growth rate and gain almost 0.5mm in length per day. Blue striped grunt, its common name, came about by the practice of making grunting sounds by grinding its pharyngeal teeth. The sound is amplified by the swim bladder acting as a resonator (Bester, 2016). DISTRIBUTION. From South Carolina, south to Brazil, consisting of the Caribbean Sea, Bermuda and Gulf of Mexico, the blue-striped grunt can be found in the western Atlantic Ocean (Fig. 2) (Bester, 2016). HABITAT AND ECOLOGY. H. sciurus are found mainly in mangroves, reefs and seagrass beds about 30m in depth (Bester, 2016). The young ones are most common in shallow water. Adult blue UWI The Online Guide to the Animals of Trinidad and Tobago Diversity striped grunts can be found in either small or large groups (Fig. -
St. Lucie, Units 1 and 2
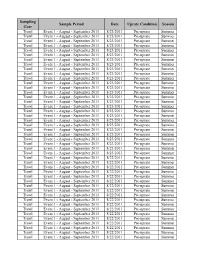
Sampling Sample Period Date Uprate Condition Season Gear Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event -

Fishery Conservation and Management Pt. 622, App. A
Fishery Conservation and Management Pt. 622, App. A vessel's unsorted catch of Gulf reef to complete prohibition), and seasonal fish: or area closures. (1) The requirement for a valid com- (g) South Atlantic golden crab. MSY, mercial vessel permit for Gulf reef fish ABC, TAC, quotas (including quotas in order to sell Gulf reef fish. equal to zero), trip limits, minimum (2) Minimum size limits for Gulf reef sizes, gear regulations and restrictions, fish. permit requirements, seasonal or area (3) Bag limits for Gulf reef fish. closures, time frame for recovery of (4) The prohibition on sale of Gulf golden crab if overfished, fishing year reef fish after a quota closure. (adjustment not to exceed 2 months), (b) Other provisions of this part not- observer requirements, and authority withstanding, a dealer in a Gulf state for the RD to close the fishery when a is exempt from the requirement for a quota is reached or is projected to be dealer permit for Gulf reef fish to re- reached. ceive Gulf reef fish harvested from the (h) South Atlantic shrimp. Certified Gulf EEZ by a vessel in the Gulf BRDs and BRD specifications. groundfish trawl fishery. [61 FR 34934, July 3, 1996, as amended at 61 FR 43960, Aug. 27, 1996; 62 FR 13988, Mar. 25, § 622.48 Adjustment of management 1997; 62 FR 18539, Apr. 16, 1997] measures. In accordance with the framework APPENDIX A TO PART 622ÐSPECIES procedures of the applicable FMPs, the TABLES RD may establish or modify the follow- TABLE 1 OF APPENDIX A TO PART 622Ð ing management measures: CARIBBEAN CORAL REEF RESOURCES (a) Caribbean coral reef resources.