An Evaluation of Two Seedling Phenotyping Protocols to Assess
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Taxonomic Revision of Rhododendron L. Section Pentanthera G
A TAXONOMIC REVISION OF RHODODENDRON L. SECTION PENTANTHERA G. DON (ERICACEAE) BY KATHLEEN ANNE KRON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 1987 , ACKNOWLEDGMENTS I gratefully acknowledge the supervision and encouragement given to me by Dr. Walter S. Judd. I thoroughly enjoyed my work under his direction. I would also like to thank the members of my advisory committee, Dr. Bijan Dehgan, Dr. Dana G. Griffin, III, Dr. James W. Kimbrough, Dr. Jonathon Reiskind, Dr. William Louis Stern, and Dr. Norris H. Williams for their critical comments and suggestions. The National Science Foundation generously supported this project in the form of a Doctoral Dissertation Improvement Grant;* field work in 1985 was supported by a grant from the Highlands Biological Station, Highlands, North Carolina. I thank the curators of the following herbaria for the loan of their material: A, AUA, BHA, DUKE, E, FSU, GA, GH, ISTE, JEPS , KW, KY, LAF, LE NCSC, NCU, NLU NO, OSC, PE, PH, LSU , M, MAK, MOAR, NA, , RSA/POM, SMU, SZ, TENN, TEX, TI, UARK, UC, UNA, USF, VDB, VPI, W, WA, WVA. My appreciation also is offered to the illustrators, Gerald Masters, Elizabeth Hall, Rosa Lee, Lisa Modola, and Virginia Tomat. I thank Dr. R. Howard * BSR-8601236 ii Berg for the scanning electron micrographs. Mr. Bart Schutzman graciously made available his computer program to plot the results of the principal components analyses. The herbarium staff, especially Mr. Kent D. Perkins, was always helpful and their service is greatly appreciated. -

Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- ERICACEAE
Flora of the Carolinas, Virginia, and Georgia, Working Draft of 17 March 2004 -- ERICACEAE ERICACEAE (Heath Family) A family of about 107 genera and 3400 species, primarily shrubs, small trees, and subshrubs, nearly cosmopolitan. The Ericaceae is very important in our area, with a great diversity of genera and species, many of them rather narrowly endemic. Our area is one of the north temperate centers of diversity for the Ericaceae. Along with Quercus and Pinus, various members of this family are dominant in much of our landscape. References: Kron et al. (2002); Wood (1961); Judd & Kron (1993); Kron & Chase (1993); Luteyn et al. (1996)=L; Dorr & Barrie (1993); Cullings & Hileman (1997). Main Key, for use with flowering or fruiting material 1 Plant an herb, subshrub, or sprawling shrub, not clonal by underground rhizomes (except Gaultheria procumbens and Epigaea repens), rarely more than 3 dm tall; plants mycotrophic or hemi-mycotrophic (except Epigaea, Gaultheria, and Arctostaphylos). 2 Plants without chlorophyll (fully mycotrophic); stems fleshy; leaves represented by bract-like scales, white or variously colored, but not green; pollen grains single; [subfamily Monotropoideae; section Monotropeae]. 3 Petals united; fruit nodding, a berry; flower and fruit several per stem . Monotropsis 3 Petals separate; fruit erect, a capsule; flower and fruit 1-several per stem. 4 Flowers few to many, racemose; stem pubescent, at least in the inflorescence; plant yellow, orange, or red when fresh, aging or drying dark brown ...............................................Hypopitys 4 Flower solitary; stem glabrous; plant white (rarely pink) when fresh, aging or drying black . Monotropa 2 Plants with chlorophyll (hemi-mycotrophic or autotrophic); stems woody; leaves present and well-developed, green; pollen grains in tetrads (single in Orthilia). -

Towards Broader Adaptability of North American Deciduous Azaleas Alexander Q
Towards Broader Adaptability of North American Deciduous Azaleas Alexander Q. Susko, James M. Bradeen, and Stan C. Hokanson orth American deciduous azaleas have and many shades in between, providing tremen- long been adored by horticulturists. dous spring and summer interest in the garden. NThese plants, which belong to the Quickly recognized for their horticultural merit, genus Rhododendron sect. [section] Pentan- North American deciduous azaleas piqued the thera, comprise 15 species distributed from interest of plant collectors upon their discovery Texas to Florida, extending northwards to and continue to be widely lauded by gardeners southern Maine, and with one species occurring today. This has led to a proliferation of cultivars in mountainous areas of Oregon and California. and interspecific hybrids that provide a beauti- These species display a wide range of flower ful floral display every year both in gardens and color, from pure white to deep orange, pink, in the wild (Azalea Society of America 2016). WILLIAM (NED) FRIEDMAN As a common name, “rhododendron” usually refers to large evergreen shrubs whose flowers have ten stamens, such as this elepidote cultivar ‘Album Grandiflorum’ (accession 22810-A) growing in the Arnold Arboretum’s Rhododendron Dell. 16 Arnoldia 74/2 • October 2016 Over 240 unique accessions of Rhododendron sect. Pentanthera exist at the Arnold Arbore- tum including many interspecific hybrids, vari- The Linnaean system classifies organisms into ous cultivars, and 12 of the 15 deciduous azalea increasingly narrow groups until reaching the species native to North America (Table 1). The individual species level. Classification (to the accessions at the Arnold Arboretum have ori- section level) for the North American deciduous gins in a wide range of environments and could azaleas is shown here (US-GRIN 2016). -

The Red List of Rhododendrons
The Red List of Rhododendrons Douglas Gibbs, David Chamberlain and George Argent BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) is a membership organization linking botanic gardens in over 100 countries in a shared commitment to biodiversity conservation, sustainable use and environmental education. BGCI aims to mobilize botanic gardens and work with partners to secure plant diversity for the well-being of people and the planet. BGCI provides the Secretariat for the IUCN/SSC Global Tree Specialist Group. Published by Botanic Gardens Conservation FAUNA & FLORA INTERNATIONAL (FFI) , founded in 1903 and the International, Richmond, UK world’s oldest international conservation organization, acts to conserve © 2011 Botanic Gardens Conservation International threatened species and ecosystems worldwide, choosing solutions that are sustainable, are based on sound science and take account of ISBN: 978-1-905164-35-6 human needs. Reproduction of any part of the publication for educational, conservation and other non-profit purposes is authorized without prior permission from the copyright holder, provided that the source is fully acknowledged. Reproduction for resale or other commercial purposes is prohibited without prior written permission from the copyright holder. THE GLOBAL TREES CAMPAIGN is undertaken through a partnership between FFI and BGCI, working with a wide range of other The designation of geographical entities in this document and the presentation of the material do not organizations around the world, to save the world’s most threatened trees imply any expression on the part of the authors and the habitats in which they grow through the provision of information, or Botanic Gardens Conservation International delivery of conservation action and support for sustainable use. -

Native Plants for Wildlife Habitat and Conservation Landscaping Chesapeake Bay Watershed Acknowledgments
U.S. Fish & Wildlife Service Native Plants for Wildlife Habitat and Conservation Landscaping Chesapeake Bay Watershed Acknowledgments Contributors: Printing was made possible through the generous funding from Adkins Arboretum; Baltimore County Department of Environmental Protection and Resource Management; Chesapeake Bay Trust; Irvine Natural Science Center; Maryland Native Plant Society; National Fish and Wildlife Foundation; The Nature Conservancy, Maryland-DC Chapter; U.S. Department of Agriculture, Natural Resource Conservation Service, Cape May Plant Materials Center; and U.S. Fish and Wildlife Service, Chesapeake Bay Field Office. Reviewers: species included in this guide were reviewed by the following authorities regarding native range, appropriateness for use in individual states, and availability in the nursery trade: Rodney Bartgis, The Nature Conservancy, West Virginia. Ashton Berdine, The Nature Conservancy, West Virginia. Chris Firestone, Bureau of Forestry, Pennsylvania Department of Conservation and Natural Resources. Chris Frye, State Botanist, Wildlife and Heritage Service, Maryland Department of Natural Resources. Mike Hollins, Sylva Native Nursery & Seed Co. William A. McAvoy, Delaware Natural Heritage Program, Delaware Department of Natural Resources and Environmental Control. Mary Pat Rowan, Landscape Architect, Maryland Native Plant Society. Rod Simmons, Maryland Native Plant Society. Alison Sterling, Wildlife Resources Section, West Virginia Department of Natural Resources. Troy Weldy, Associate Botanist, New York Natural Heritage Program, New York State Department of Environmental Conservation. Graphic Design and Layout: Laurie Hewitt, U.S. Fish and Wildlife Service, Chesapeake Bay Field Office. Special thanks to: Volunteer Carole Jelich; Christopher F. Miller, Regional Plant Materials Specialist, Natural Resource Conservation Service; and R. Harrison Weigand, Maryland Department of Natural Resources, Maryland Wildlife and Heritage Division for assistance throughout this project. -

The Vascular Flora of Sandy Run Savannas State Natural Area, Onslow and Pender Counties, North Carolina --In Press-- John B
The Vascular Flora of Sandy Run Savannas State Natural Area, Onslow and Pender Counties, North Carolina --In Press-- John B. Taggart Department of Environmental Studies, University of North Carolina at Wilmington, 601 South College Road, Wilmington, North Carolina 28403 ______________________________________________________________________________ ABSTRACT The vascular plants of Sandy Run Savannas State Natural Area, located in portions of Onslow and Pender counties, North Carolina, are presented as an annotated species list. A total of 590 taxa in 315 genera and 119 families were collected from eight plant communities. Families with the highest numbers of species were the Asteraceae (80), Poaceae (66), and Cyperaceae (65). Two species, Carex lutea (golden sedge) and Thalictrum cooleyi (Cooley’s meadowrue), have federal endangered status. A total of 23 taxa are tracked by the North Carolina Natural Heritage Program, while 29 others are considered rare, but not included on the priority list. Of 44 species considered strict endemic or near-endemic taxa to the North and South Carolina Coastal Plain, 18 (41%) were collected in this study. Selected pine savannas within the site were rated as nationally significant by the North Carolina Natural Heritage Program. Fifty-one (51) non-native species were present and represented 8.7 % of the flora. _________________________________________________________________________ INTRODUCTION Sandy Run Savannas State Natural Area encompasses portions of western Onslow and northeastern Pender counties in North Carolina. State acquisition of this coastal plain site began in 2007 as a cooperative effort between The Nature Conservancy in North Carolina and the North Carolina Division of Parks and Recreation to protect approximately 1,214 ha comprised of seven tracts (Figure 1). -

Volume 9, Number 1, March 1987
THE AZALEAN Journal of the Azalea Society of America Volume 9 Number 1 March 1987 AZALEA SOCIETY OF AMERICA The Azalea Society of America, organized December 9, 1977 and incorporated in the District of Columbia, is an educational and scientific non-profit association devoted to the culture, propagation and appreciation of the series Azalea (subgenus Anthodendron) of the genus Rhododendron in the Heath family (Ericaceae). OFFICERS FOR 1986-1987 PRESIDENT - Ryon A. Page VICE-PRESIDENT - Eleanor Stubbs (Silver Spring, Maryland) (West Linn, Oregon) SECRETARY - Valerie Lorenz TREASURER - Glenn W. Taylor (Fairfax Station, Virginia) (Springfield, Virginia) IMMEDIATE PAST-PRESIDENT - John U. Rochester, Jr. (Franklinton, Louisiana) BOARD OF GOVERNORS Terms expiring in 1987 Terms expiring in 1988 Chapter presidents serve as ex-officio members James A. (Tony) Dove, Jr. L. Malcolm Clark (chairman) Charles H. Evans, M.D., Ph.D. Fred C. Galle Donald W. Hyatt David Lay Ryon A. Page Robert T. Stelloh Russell L. Scott Donald H. Voss CHAPTERS Brookside Gardens (chartered August 1979) Ralph W. Pennington (chartered June 1981) William L. Clagett, president Roy Kelly, president Richmond, Virginia (chartered August 1979) Tri-State (chartered October 1981) Page Calisch, president Lloyd Hahn, president Robert D. Gartrell (chartered May 1980) Mobile (chartered March 1983) Jerry Goodman, president Pat Ryan, president Ben Morrison (chartered May 1980) Northwest (chartered October 1983) Robert Hobbs, president Eleanor Stubbs, president Northern Virginia (chartered May 1980) Flame Azalea (chartered May 1984) Betty Jones, president Allen Cantrell, president Louisiana (chartered June 1981) Delmarva (chartered May 1986) John U. Rochester, Jr., president Gordon W. Severe, president Regular membership is open to all interested parties for an annual contribution of $15.00. -

Rare Vascular Plant Taxa Associated with the Longleaf Pine Ecosystems: Patterns in Taxonomy and Ecology
Rare Vascular Plant Taxa Associated with the Longleaf Pine Ecosystems: Patterns in Taxonomy and Ecology Joan Walker U.S.D.A. Forest Service, Southeastern Forest Experiment Station, Department of Forest Resources, Clemson University, Clemson, SC 29634 ABSTRACT Ecological, taxonomic and biogeographical characteristics are used to describe the group of 187 rare vascular plant taxa associated with longleaf pine (Pinus palustris) throughout its range. Taxonomic and growth form distributions mirror the patterns of common plus rare taxa in the flora. Most of the species have rather narrow habitat preferences, and narrow geo graphic ranges, but a few rare sp~cies with broad habitat tolerances and wider geographic ranges are identified. Ninety-six local endemics are associated with longleaf pine ecosystems. This incidence is as high as in other comparably-sized endemic-rich areas in North America. A distinct geographic trend in rare species composition is indicated. Species fall into 4 groups: Florida longleaf associates, south Atlantic coastal plain, east Gulf coastal plain, and west Gulf coastal plain species. Distributional factors that produce rarity must be considered in the development of conser vation strategies. Overall, conserving longleaf communities rangewide will protect .large ~ numbers of rare plant taxa in Southeastern United States. INTRODUCTION 1986), and inevitably the strategies required to con serve them will differ. Recently Hardin and White (1989) effectively focused conservationists' attentions on the high The purposes of this study are to (1) identify numbers of rare species associated with wiregrass the rare species associated with longleaf pine eco (Aristida stricta), a grass that dominates the ground systems rangewide; (2) characterize the rare spe layer of longleaf communities through a large part cies taxonomically and ecologically, in order to of its range, and over a broad range of longleaf identify patterns that may distinguish this group habitats. -

JOURNAL AMERICAN RHODODENDRON SOCIETY 231 Evolution, Adaptive Radiation and Vireya — a Review
Index Journal American Rhododendron Society, ‘Rebecca Taffet’. 4:232. Volume 66, 2012 ‘Rosebud’. 1:46. The names of species and hybrid ‘Sandra’s Green Ice’. 1:42. rhododendrons and azaleas occurring ‘Washington State Centennial’. 1:46. within the text of articles are not listed ‘White Find’. 1:43. in the index. Reference to plants with colour images is made for the following AZALEA SPECIES AND SELECTED entries: (AZALEA HYBRIDS, AZALEA SPECIES FORMS SPECIES, RHODODENDRON HYBRIDS, R. albiforum. 4:198 RHODODENDRON SPECIES, VIREYA R. austrinum. 3:135. HYBRIDS, VIREYA SPECIES). R. calendulaceum. 1:42; 4:183, 186. R. prunifolium. 3:135. 2011: An Unusual Weather Year in Georgia. R. vaseyi. 1:18. Charles Andrews. 3:135-136. R. viscosum var. aemulans.3:126. A Breeding Program for Zone 6 Big Leaf R. viscosum var. serrulatum.3:123. Rhododendrons. Bruce Clyburn. 3:159- 162. Beck, Carolyn. Hooper Bald Native Azalea A Novel Propagation Unit for Rooting Project. 4:183-186. Rhododendron Cuttings. Marc Bottemiller, Dennis. Variables Involved in Colombel. 3:157-158. Rooting Rhododendron Cuttings. 2:68- A Rhododendron Untamed: R. albiforum. 70. Peter Kendall. 4:198-200. Campbell, Alan. Hybrids of Haida Gold Adams, Peter. Evolution, Adaptive Radiation Gardens. 2:108, 110. and Vireya Rhododendrons, Part I. Cheatle, Lee. Rhododendrons and Azaleas 4:201-203. as Bonsai. 2:71-72. Alies’ Pliers. Marc Colombel, 4:238. Clyburn, Bruce. A Breeding Program for Andrews, Charles.The Biltmore Estate’s Zone 6 Big Leaf Rhododendrons. Rhododendron Legacy. 4:213-215. 3:159-162. Andrews, Charles. 2011: An Unusual Cockeram, Gene. ‘The Pink Ribbons’ Weather Year in Georgia. -
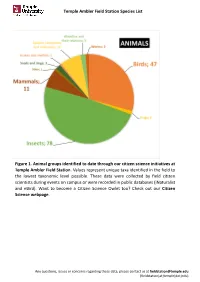
Temple Ambler Field Station Species List Figure 1. Animal Groups Identified to Date Through Our Citizen Science Initiatives at T
Temple Ambler Field Station Species List Figure 1. Animal groups identified to date through our citizen science initiatives at Temple Ambler Field Station. Values represent unique taxa identified in the field to the lowest taxonomic level possible. These data were collected by field citizen scientists during events on campus or were recorded in public databases (iNaturalist and eBird). Want to become a Citizen Science Owlet too? Check out our Citizen Science webpage. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List Figure 2. Plant diversity identified to date in the natural environments and designed gardens of the Temple Ambler Field Station and Ambler Arboretum. These values represent unique taxa identified to the lowest taxonomic level possible. Highlighted are 14 of the 116 flowering plant families present that include 524 taxonomic groups. A full list can be found in our species database. Cultivated specimens in our Greenhouse were not included here. Any questions, issues or concerns regarding these data, please contact us at [email protected] (fieldstation[at}temple[dot]edu) Temple Ambler Field Station Species List database_title Temple Ambler Field Station Species List last_update 22October2020 description This database includes all species identified to their lowest taxonomic level possible in the natural environments and designed gardens on the Temple Ambler campus. These are occurrence records and each taxon is only entered once. This is an occurrence record, not an abundance record. IDs were performed by senior scientists and specialists, as well as citizen scientists visiting campus. -

Pollinator Adaptation and the Evolution of Floral Nectar Sugar
doi: 10.1111/jeb.12991 Pollinator adaptation and the evolution of floral nectar sugar composition S. ABRAHAMCZYK*, M. KESSLER†,D.HANLEY‡,D.N.KARGER†,M.P.J.MULLER€ †, A. C. KNAUER†,F.KELLER§, M. SCHWERDTFEGER¶ &A.M.HUMPHREYS**†† *Nees Institute for Plant Biodiversity, University of Bonn, Bonn, Germany †Institute of Systematic and Evolutionary Botany, University of Zurich, Zurich, Switzerland ‡Department of Biology, Long Island University - Post, Brookville, NY, USA §Institute of Plant Science, University of Zurich, Zurich, Switzerland ¶Albrecht-v.-Haller Institute of Plant Science, University of Goettingen, Goettingen, Germany **Department of Life Sciences, Imperial College London, Berkshire, UK ††Department of Ecology, Environment and Plant Sciences, University of Stockholm, Stockholm, Sweden Keywords: Abstract asterids; A long-standing debate concerns whether nectar sugar composition evolves fructose; as an adaptation to pollinator dietary requirements or whether it is ‘phylo- glucose; genetically constrained’. Here, we use a modelling approach to evaluate the phylogenetic conservatism; hypothesis that nectar sucrose proportion (NSP) is an adaptation to pollina- phylogenetic constraint; tors. We analyse ~ 2100 species of asterids, spanning several plant families pollination syndrome; and pollinator groups (PGs), and show that the hypothesis of adaptation sucrose. cannot be rejected: NSP evolves towards two optimal values, high NSP for specialist-pollinated and low NSP for generalist-pollinated plants. However, the inferred adaptive process is weak, suggesting that adaptation to PG only provides a partial explanation for how nectar evolves. Additional factors are therefore needed to fully explain nectar evolution, and we suggest that future studies might incorporate floral shape and size and the abiotic envi- ronment into the analytical framework. -
Catalog.Shrub & Tree Catalog Edited
Willis Farm Shrubs & Trees, Perennials & Fruits Catalogs 1. Plants are arranged in alphabetical order by binomial name. 2. Photographs of selected plant names below can be viewed by clicking on the blue colored plant names. 3. This list includes plants that we grow. Please call for availability. Shrubs & Trees: Please Note that We Do Not Grow All Plants in this Catalog at Any One Time. We Leave Notes Here For Your Information. Please Call for Availability. Call or Text 318-210-4507, 8 am to 4 pm Monday to Friday Maples: Family: Sapindaceae (Soapberry) NATIVE MAPLES: Acer Barbatum / (southern sugar maple) Designated as Louisiana Super Plant by LSA Ag. 30’ - 45’ Deciduous tree, with good, generally yellow, to yellow-orange fall color in south. Wildlife: Yellow bellied sapsucker, seed eaten by song sparrows and orioles. Culture: Z7-9, Prefers sun, some shade OK, likes rich well-drained, but not dry, soil. Adaptable to most soil conditions including basic soils. Likes slopes, tolerates heat & humidity. Moderate to fast growth. Medium spread. Native to Southeastern USA. Acer rubrum ‘Brandywine’ / (brandywine red maple) Brilliant red-purple fall color. Cross of A rubrum ‘October Glory’ and ‘Autumn Flame’. Deciduous tree grows to 40’, prefers slightly acid soil but adaptable. Introduction of National Arboretum. A Male selection so no weedy seedlings. Leafhopper resistance is significant, a major problem of landscape maples. Culture: Z4-8, full sun to partial shade, OK in wet or dry areas, needs acid soil for good health and best color, but fairly tolerant of wide range of soil conditions. Propagation: Easily from softwood cuttings under mist, 1-3000 IBA.