COVID-19: Vaccine Storage and Handling Guidance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Brunswick COVID-19 Vaccine Clinic Guide for Immunizers
New Brunswick COVID-19 Vaccine Clinic Guide for Immunizers Department of Health Public Health New Brunswick September 17, 2021 Public Health New Brunswick New Brunswick Department of Health PO Box 5100 Fredericton, New Brunswick, E3B 5G8 Canada This report is available online: www.gnb.ca/publichealth Ce document est aussi disponible en français sur le titre «Guide sur la vaccination contre la COVID-19 pour les Vaccinateurs du Nouveau-Brunswick ». TABLE OF CONTENTS NEW BRUNSWICK COVID-19 CLINIC GUIDE FOR IMMUNIZERS .......................................................... 1 1.0. PURPOSE ....................................................................................................................................... 1 2.0. VACCINE SOP ON TRANSPORTATION AND RECEIPT ............................................................. 1 Vaccines – storage and handling and maintaining cold chain to prevent temperature excursions (portable freezers and refrigeration) .......................................................... 2 3.0. SCHEDULES, DOSES AND ADMINISTRATION OF COVID-19 VACCINES ............................... 3 Carton Labelling of COVID-19 Vaccines ........................................................................ 4 4.0. REDUCING UNNECESSARY VACCINE WASTAGE .................................................................... 4 Context .............................................................................................................................. 4 Planning and prioritizing individual vaccination over wastage ................................. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
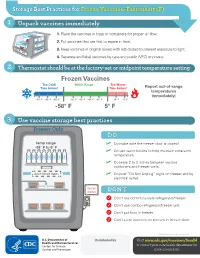
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

CARPHA COVID-19 Vaccine Update 015 April 19, 2021
CARPHA UPDATE FOR Incident Manager / SITUATION REPORT COVID-19 Vaccines Update Supplement Week of: 19th April, 2021 I. Overview of Development and Regulatory Approvals: • 88 candidate vaccines are in clinical development: 16 in Phase 3 trials, and 4 in Phase 4 trials – see Figure in CARPHA COVID-19 Vaccine Regulatory Tracker (Phases tab). • 15 vaccines have received regulatory approvals in various countries, and 18 vaccines are at various stages of engagement with WHO for emergency use listing (EUL). • One additional AstraZeneca vaccine was approved by the WHO for Emergency Use Listing on 15th April, bringing the total number of listed COVID-19 vaccines by different manufacturers to 5: Pfizer-BioNTech’s vaccine: COMIRNATY®, AstraZeneca-SK Bio, AstraZeneca-SII (Covishield), Janssen-Cilag, AstraZeneca (EU node; Vaxzevria™) • 2 additional vaccines are expected to be approved by WHO in April, and 1 in May: Tables 1 and 3. • There is one additional vaccine being considered by WHO however it is at the stage of submitting expressions of interest: Bayer AG – Germany (CureVAC) • The WHO Global Advisory Committee on Vaccine Safety (GACVS) issued an interim statement indicating that based on current information, a causal relationship between the vaccine and the occurrence of rare blood clots with low platelets is considered plausible but is not confirmed. Specialised studies are needed to fully understand the potential relationship between vaccination and possible risk factors. The WHO maintains that the benefit-risk balance of the vaccine remains favorable. • CARPHA has shared its COVID-19 vaccine regulatory tracker with Member States for viewing as updates are made. • CARPHA-CRS has completed its review of the COVID-19 vaccine by Janssen-Cilag Pharmaceuticals (Johnson & Johnson), which will be finalized and shared with chief medical officers this week. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

COVID-19 Vaccine Storage & Handling
COVID-19 Vaccine Storage & Handling Brooke Zeringue, Adrienne Whitney, & Robert Starszak Louisiana Department of Health Office of Public Health Immunization Program • Everyone (16 years and older) can be vaccinated. Louisiana Order COVID-19 vaccine in LINKS. Second COVID-19 doses are not automatically ordered. You must Vaccination order every dose you need. Guidelines All vaccine doses administered must be entered into LINKS within 24 hours and inputted as administered, NOT historical. COVID-19 Vaccine Delivery Storage & Handling for Moderna COVID-19 Vaccine: Direct from McKesson Vaccine will arrive frozen Larger quantities Thermal Shipper Storage & Handling for Pfizer- BioNTech COVID-19 Vaccine: Direct from Shipped in thermal shipping container Pfizer Vaccine arrives frozen Larger quantities Storage & Handling for Moderna COVID-19 Vaccine: Morris & Dickson Vaccine will arrive thawed Smaller Quantities Johnson & Johnson (Janssen) COVID-19 Vaccine Vaccine Type: • Viral Vector Vaccine Age indication: Overview of • 18 years or older. the Johnson & Dose & Route: Johnson • 0.5 mL; intramuscular COVID-19 Schedule: Vaccine • Single dose Dose Preparation: • 5 doses per vial Johnson & Johnson | STORING VACCINE VIALS Refrigerator 2°C to 8°C (36°F to 46°F) • Store up to the expiration date • DO NOT store frozen • Protect from light • You can find the expiration date at vaxcheck.jnj Johnson & Johnson | ADMINISTERING • Keep refrigerated until thawed (if arrived frozen). Refrigerator • PUNCTURED VIALS: Viable up to 6 hours 2°C to 8°C (36°F to 46°F) • If arrived frozen and needed immediately, thaw for Room 1 to 2 hours. • UNPUNCTURED VIALS: Viable up to 12 hours kept Temperature at 9°C to 25°C (47°F to 77°F) • PUNCTURED VIALS: Viable up to 2 hours Up to 25°C (Up to 77°F) Johnson & Johnson | ADMINISTERING Visually inspect the Before withdrawing each Johnson & Johnson COVID- dose of vaccine, carefully 19 vaccine vials for other mix the contents by Each dose must contain 0.5 particulate matter and/or swirling gently in an upright mL of vaccine. -

Overview of the Implementation of COVID-19 Vaccination Strategies and Deployment Plans in the EU/EEA
[Type here] TECHNICAL REPORT Overview of the implementation of COVID-19 vaccination strategies and deployment plans in the EU/EEA 14 June 2021 Key messages This report provides an updated overview of the progress of national COVID-19 vaccination strategies in EU/EEA countries, including updates on: • overall vaccine uptake and uptake by target group; • current vaccination phases and priority groups, including adjustments made to priority groups during the rollout; • vaccination strategies and policies; • the use of vaccination certificates; • vaccine acceptance and hesitancy; and • challenges and good practice with the rollout. Vaccine COVID-19 rollout overview • As of 11 June 2021, a total of 333 678 903 COVID-19 vaccine doses have been distributed by manufacturers to European Union/European Economic Area (EU/EEA) countries, including over 39 million in the last week. Comirnaty (BNT162b2), developed by BioNTech/Pfizer, represents 67.3% of all doses distributed to EU/EEA countries via the European Commission’s Vaccine Strategy, followed by Vaxzevria (AZD1222), previously called COVID-19 Vaccine AstraZeneca (19.5%), COVID-19 Vaccine Moderna (9.6%), and COVID-19 Vaccine Janssen (3.3%). • A total of 284 124 689 vaccine doses have been administered in the EU/EEA, including over 25 million in the last week. Based on data available from 29 countries, 85% of the doses distributed in the EU/EEA since the beginning of the rollout have been administered. • Since the start of COVID-19 vaccine deployment in the EU/EEA in December 2020, the cumulative vaccine uptake in the adult population (aged 18 years and older) in the EU/EEA has progressed, reaching 51.2% for at least one vaccine dose (range: 14.9-67.7%) and 26.8% for the full vaccination course (range: 11.8-55.1%) (30 reporting countries). -

Responsive COVID-19 Vaccines for Recovery Project Under the Asia Pacific Vaccine Access Facility (Guaranteed by the Republic of Indonesia)
Report and Recommendation of the President to the Board of Directors Project Number: 54425-001 March 2021 Proposed Loan PT Bio Farma (Persero) Responsive COVID-19 Vaccines for Recovery Project under the Asia Pacific Vaccine Access Facility (Guaranteed by the Republic of Indonesia) Distribution of this document is restricted until it has been approved by the Board of Directors. Following such approval, ADB will disclose the document to the public in accordance with ADB’s Access to Information Policy. CURRENCY EQUIVALENTS (as of 5 March 2021) Currency unit – rupiah (Rp) Rp1.00 = $0.0000697 $1.00 = Rp14,349 ABBREVIATIONS ADB – Asian Development Bank AEFI – adverse event following immunization APVAX – Asia Pacific Vaccine Access Facility Bio Farma – PT Bio Farma (Persero) COVID-19 – coronavirus disease Indofarma – PT Indofarma Tbk LIBOR – London interbank offered rate M&E – monitoring and evaluation MOH – Ministry of Health PAM – project administration manual RRC – rapid response component TA – technical assistance UNICEF – United Nations Children’s Fund VAP – Vaccination Allocation Plan VIRAT – Vaccination Introduction Readiness Assessment Tool WHO – World Health Organization NOTE In this report, “$” refers to United States dollars. Vice-President Ahmed M. Saeed, Operations 2 Director General Ramesh Subramaniam, Southeast Asia Department (SERD) Directors Ayako Inagaki, Human and Social Development Division (SEHS), SERD Winfried Wicklein, Country Director, Indonesia Resident Mission (IRM), SERD Said Zaidansyah, Deputy Country Director, IRM, -

Optimal SARS-Cov-2 Vaccine Allocation Using Real-Time Seroprevalence Estimates in Rhode Island and Massachusetts
medRxiv preprint doi: https://doi.org/10.1101/2021.01.12.21249694; this version posted January 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license . Optimal SARS-CoV-2 vaccine allocation using real-time seroprevalence estimates in Rhode Island and Massachusetts Thu Nguyen-Anh Tran1, Nathan Wikle2, Joseph Albert3, Haider Inam4, Emily Strong2, Karel Brinda5;6, Scott M Leighow4, Fuhan Yang1, Sajid Hossain7, Justin R Pritchard4, Philip Chan8, William P Hanage5, Ephraim M Hanks2, Maciej F Boni1 1 Center for Infectious Disease Dynamics, Department of Biology, Pennsylvania State University, University Park, PA 2 Center for Infectious Disease Dynamics, Department of Statistics, Pennsylvania State University, University Park, PA 3 Department of Physics, Pennsylvania State University, University Park, PA 4 Center for Infectious Disease Dynamics, Department of Bioengineering, Pennsylvania State University, University Park, PA 5 Center for Communicable Disease Dynamics, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, MA 6 Department of Biomedical Informatics, Harvard Medical School, Boston, MA 7 Yale School of Medicine, Yale University, New Haven, CT 8 Department of Medicine, Brown University, Providence, RI Running Title: SARS-CoV-2 vaccination in RI and MA Keywords: SARS-CoV-2, vaccination, optimal vaccine allocation, mathematical modeling, real-time seroprevalence Date of this draft : January 12, 2021 ?Manuscript Correspondence: Maciej F. Boni Center for Infectious Disease Dynamics Department of Biology Pennsylvania State University University Park, PA tel +1 814 867 4651 email: [email protected] 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

June 2021 2019
CURRENT AFFAIRS ORGANIC AND ORGANISED DECEMBERJUNE 2021 2019 A LETTER FROM MY HEART Dear IAS Aspirant Friends, It gives me immense pleasure to present to you the 360º Current Affairs Magazine for the month of June 2021. The dedicated team that compiles and edits Current Affairs at IAS WINNISHERS has made sincere efforts to provide to you the most relevant and important news from the point of view of Interview, Mains and especially the soon approaching Prelims. Our mission is to build IAS aspirants into human beings who can become IAS officers. In that direction, we strive to facilitate the current affairs knowledge that is ORGANIC and ORGANISED. Due to the ongoing unfortunate situation, we fully empathize with your anxiety related to the exam. This compilation aids you in your preparation, especially the soon approaching Prelims exam. This issue also carries information on INTERVIEW GUIDANCE PROGRAM conducted by IAS WINNISHERS, which has produced amazing results in the past. Get more information on our website and benefit immensely from it. Wishing You Success Vinay Kumar R Founder & CEO, IAS WINNISHERS Vinay Kumar R International NLP & IAS Coach 9036113902 | 9886273325 www.iaswinnishers.com © Winnishers Educational Services Pvt Ltd © Winnishers Educational Services Pvt Ltd 1 Contents 1. POLITY & CONSTITUTION ............................................................................................................ 8 1.1.LAST ‘D-VOTER’ WALKS OUT OF ASSAM DETENTION CENTRE ................................................................ -

Dutch Statutory Board Report and Financial Statements of Curevac N.V
Dutch statutory board report and financial statements of CureVac N.V. for the fiscal year ended December 31, 2020 Table of Contents Dutch Statutory Board Report ......................................................................................................... 3 1. Introduction......................................................................................................................................... 3 1.1 Preparation ................................................................................................................................... 3 1.2 Forward-Looking Statements .............................................................................................. 3 2. Company and business overview .............................................................................................. 5 2.1 History and development of the Company .................................................................... 5 2.2 Business overview .................................................................................................................... 5 2.3 Organizational Structure ................................................................................................... 120 2.4 Property, Plant and Equipment....................................................................................... 120 2.5 Material subsequent events ............................................................................................. 123 3. Financial Overview ...................................................................................................................... -

2021 Vaccine Storage and Handling
2021 Vaccine Storage and Handling Maine Immunization Program Annual Education Requirement Vaccines for Children Learning Objectives Learning Objectives At the conclusion of this training, the participant will be able to: 1. Define and explain cold chain management. 2. Describe the components of routine and emergency procedures for vaccine storage and handling. 3. Describe the roles of the primary and backup coordinators and other staff in the storage and handling of vaccines. 4. Describe proper vaccine storage and temperature monitoring equipment. 5. Describe correct vaccine and diluent storage, handling, and disposal of routinely recommended vaccines. 6. Identify actions that should be taken if vaccines have not been stored properly. Maine Center for Disease Control and Prevention 2 Introduction Proper vaccine storage and handling has been an important factor in preventing and eradicating many common vaccine-preventable diseases. Yet, each year, storage and handling errors result in revaccination of many patients and significant financial loss due to wasted vaccine. Failure to store and handle vaccines properly can reduce vaccine potency, resulting in inadequate immune response in patients and poor protection against disease. Patients can lose confidence in vaccines and providers if they have to be revaccinated because the vaccines they have received may have been compromised. This annual education requirement provides an overview of approved vaccine storage and handling best practices. For more detailed information, refer to the CDC’s Vaccine Storage and Handling Toolkit. Maine Center for Disease Control and Prevention 3 Cold Chain The vaccine cold chain is a temperature-controlled environment used to maintain and distribute vaccines in optimal condition.