KOBE Biomedical Innovation Cluster
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
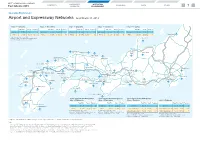
7. Airport and Expressway Networks (PDF, 352KB)
WEST JAPAN RAILWAY COMPANY CORPORATE OPERATING CONTENTS BUSINESS DATA OTHER Fact Sheets 2019 OVERVIEW ENVIRONMENT 7 Operating Environment Airport and Expressway Networks As of March 31, 2019 Tokyo — Fukuoka Tokyo — Hiroshima Tokyo — Okayama Tokyo — Kanazawa Tokyo — Toyama Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Travel Time Fare (¥) Frequency Shinkansen 4h 46m 22,950 31 Shinkansen 3h 44m 19,080 46 Shinkansen 3h 09m 17,340 60 Shinkansen 2h 28m 14,120 24 Shinkansen 2h 08m 12,730 24 Niigata Airport Airlines 3h 00m 41,390 54 (19) Airlines 3h 30m 34,890 18 Airlines 3h 10m 33,990 10 Airlines 2h 50m 24,890 10 Airlines 2h 30m 24,890 4 Travel Time and Fare: JAL or ANA Noto Airport Frequency: All airlines. Numbers in parentheses are frequency excluding those of JAL or ANA. Kanazawa Izumo Airport Komatsu Toyama Airport Yonago Airport Airport Tottori Airport Yonago Hagi Iwami Airport Izumo Tajima Airport Gotsu Hamada Tsuruga Yamaguchi Ube Airport Yamaguchi HiroshimaHiroshima Hiroshima Airport Okayama Airport Maibara Kitakyushu Ibaraki Airport Onomichi Hakata KomakiKomaki AirportAirport Okayama KobeKobe ItamiItami AirportAirport Fukuoka Airport Kitakyushu Airport KKurashikiurashiki SSuitauita Iwakuni Kintaikyo NagoyaNagoya Sasebo Tosu Airport Sakaide Shin-OsakaShin-Osaka Tokyo Saga Airport Imabari Kobe Airport Narita Airport Matsuyama Airport Takamatsu Airport Naruto KansaiKansai AirportAirport Haneda Airport Oita Airport Kansai Nagasaki International Airport Chubu International -

Activities in Japan 1 Activities in Japan
Chapter 3 Activities in Japan 1 Activities in Japan (1) Schedule Date Time Program October 27 <National Leaders (NLs), Participating Youths (PYs) and host family representatives Tuesday from ASEAN member countries> Arrival at Narita International Airport 6:45 Myanmar (NH-814) 7:15 Malaysia, Brunei Darussalam (MH-088) 7:35 Lao P.D.R., Cambodia (TG-642) 8:00 Host family representatives from Vietnam (VN-300) 8:50 Indonesia (GA-874) *arrival at Haneda airport Transfer to the Cabinet Office for orientation Move to Hotel New Otani Tokyo 15:00 Philippines (NH-820) 15:05 Vietnam (VN-384) *arrival at Haneda airport 16:05 Singapore (JL-712) 17:30 Thailand (JL-032)*arrival at Haneda airport Transfer to Hotel New Otani Tokyo and orientation at the hotel Stay at Hotel New Otani Tokyo <Japanese PYs> Pre-departure training Stay at National Olympics Memorial Youth Center October 28 <Japanese PYs> Wednesday 8:15 Move to Hotel New Otani Tokyo <NLs, PYs and host family representatives> 9:00-11:00 Orientation (“Ho-oh”, Hotel New Otani Tokyo) • Speech by Mr. Hideki Uemura, Administrator • Introduction of NLs and PYs • Introduction of host family representatives • Introduction of Administrative staff members • Explanation of the country program in Japan • Speech by Ms. Tomoko Okawara, Chairperson of Japan-ASEAN Youth Leaders Summit (YLS) Organizing Committee • Solidarity Group (SG) meeting <Host family representatives> 11:15-11:45 Courtesy call on Mr. Takahiko Yasuda, Director General for International Youth Exchange, Cabinet Office (“Tsubaki”, Hotel New Otani Tokyo) • Speech by Mr. Takahiko Yasuda, Director General for International Youth Exchange, Cabinet Office • Presentation of certificate and gift • Photo session 30 Chapter 3 Activities in Japan Date Time Program October 28 <NLs, PYs and host family representatives> Wednesday 12:00-12:30 Inauguration Ceremony (“Ho-oh”, Hotel New Otani Tokyo) • Moment of silence for the victims of the bus accident in Brunei Darussalam in 2001 • Speech by Mr. -

Times Car RENTAL
Times Car RENTAL Telephone Shop Name Business hours (Global Customer Desk) Centrair Chubu International Airport 08:00~20:00 08:00~20:00 Nagoya Station Shinkansen Extrance 08:00~22:00(Fri・Sat・Sun・National holidays and the day before、08/10~08/15、12/28~01/03) Nagoya Station 08:00~20:00 Kamimaezu 08:00~20:00 Nagoya Fushimi 08:00~21:00 Haneda Airport 08:00~21:00 Haneda Airport Terminal 1 08:00~21:00 Haneda Airport Terminal 2 08:00~21:00 Hachioji Station 08:00~20:00 08:00~20:00 Yurakucho 07:00~22:00(Fri・Sat・Sun・National holidays、04/28~05/05、08/10~08/16、11/02、11/22、12/22、12/28 ~01/03) 08:00~20:00 Shinagawa Railway Station 08:00~22:00(Fri・Sat・Sun・National holidays、04/28~05/05、08/10~08/16、11/02、11/22、12/22、12/28 ~01/03) 08:00~20:00 Shin Yokohama Prince Hotel 08:00~22:00(Fri・Sat・Sun・National holidays、04/28~05/05、08/10~08/16、11/02、11/22、12/22、12/28 +81-50-3786-0056 ~01/03) Mishima 08:00~20:00 Shin Fuji Station 08:00~20:00 Shizuoka Staion 08:00~20:00 Hamamatsu Railway Station 08:00~20:00 Gifu Station 08:00~20:00 Kyoto Station 08:00~22:00 Tottori Airport 08:00~19:00 Yonago Airport 08:00~20:00 Izumo Airport 08:00~20:00 08:00~20:00 Fukuyama Station 09:00~18:00(12/31~01/03) Hiroshima Station 08:00~20:00 08:00~20:00 Hiroshima Otemachi 09:00~19:00(12/31~01/03) 08:00~20:00 Hiroshima Station Shinkansen Entrance 08:00~21:00(07/01~09/30) 08:00~20:00 Hiroshima Airport 08:00~21:00(07/01~09/30) Shin Onomichi Station 08:00~20:00 Mihara Station 08:00~20:00 Okayama Station (West Entrance) 08:00~20:00 Kurashiki Station 08:00~20:00 Okayama Station (East Entrance) 08:00~20:00 -

Nippon Rent-A-Car
Nippon Rent-A-Car https://www.nrgroup-global.com/en/ Telephone Shop Name Business hours (ENGLISH SERVICE DESK) Shin-Aomori Station 8:00〜20:00 Aomori Station 8:00〜20:00 8:00〜19:00(3/1~11/30) Aomori Van Truck Center 8:00〜18:00(12/1~2/28) Aomori Airport 8:00〜21:30 8:00〜19:00(3/1~11/30) Goshogawara 8:00〜18:00(12/1~2/28) Hirosaki Station 8:00〜19:00 Shichinohe Towada Station 8:00〜20:00 Hachinohe Station East Exit 8:00〜20:00 Hachinohe Shiroshita 8:00〜20:00 Misawa Station 8:00〜18:00 Misawa Airport 8:00〜19:00 Misawa Airport 8:00〜19:00 +81-3-6859-6234 Mutsu Shimokita Station 8:00〜19:00 Akita Station East Exit 8:00〜20:00 8:00〜20:00(3/1~11/30) Akita Sanno Jujiro 8:00〜19:00(12/1~2/28) Akita Airport 8:00〜21:10 Omagari 8:00〜19:00 Yokote Station West Exit 8:00〜19:00 Kakunodate Station 8:00〜19:00 Odate Station 8:00〜19:00 Odate Noshiro Airport 8:00〜18:00 8:00〜19:00(3/1~11/30) Takanosu 8:00〜18:00(12/1~2/28) Higashi-Noshiro Station 8:00〜18:00 8:00~19:00(3/1~11/30) Ugohonjo Station 8:00~18:00(12/1~2/28) Morioka Station 7:00〜21:00 Morioka Bus Center 8:00〜19:00 Morioka Van Truck Center 8:00〜20:00 Morioka Minami-Senboku 8:00〜19:00 Ninohe Station West Exit 8:00〜20:00 Kuji Station East Exit 8:00〜19:00 Miyako 8:00〜19:00 Shin-Hanamaki Station East Exit 8:00〜20:00 Hanamaki Airport +81-3-6859-6234 8:00〜19:00 Kitakami Station West Exit 8:00〜20:00 Mizusawa Esashi Station 8:00〜20:00 Ichinoseki 8:00〜20:00 Kesennuma 8:00~18:00 Sendai Station Terminal 7:00〜21:00 8:00~17:00 Sendai Station West Exit ShopHpliday(Sat, Sun, National holidays, 01/01~01/03) Sendai Honcho 7:00〜20:00 -

ACCESS Takeshima Dōgo Oki Islands
ACCESS Takeshima Dōgo Oki Islands Okinoshima Town Island Saigō Port Adventure! Dōzen OKI ISLANDS Nishinoshima Oki Shimane Prefecture, Japan Town Airport 2021 See the amazing Beppu Hishiura Port Shin- Oki Islands Port Chitose scenery of the Ama Town Oki Islands! Shimane Chibu Kurii Port Shichirui Port Village 30 min 50 min 1 hr 30 min Check Tokyo it out! Narita Airport Sakaiminato Port Sendai Fukuoka ( Haneda Airport ) Yonago 1 hr 30 min Osaka Airport Itami Airport Route 9 Guide Book Matsue (Kansai Airport) Izumo Yonago Tokyo Izumo Airport 50 min 1 hr Ochiai Miyoshi Shizuoka Notice for Visitors Winter in Oki can be very cold and windy. The ferry Nagoya Fukuoka Hiroshima Okayama Osaka and airplane are sometimes canceled. We recommend JR (Shinkansen) that you visit spring-autumn when there is more to do and see. *Takeshima is not included in the geopark area. *The Shin-Chitose ー Izumo Flight is only available in August . From Tokyo From Osaka Oki Islands UNESCO Global Geopark | Daisen-Oki National Park Oki Saigō Port (all ports) Air(JAL) Saigō Port Dōzen Oki Airport (Dōgo) Itami Airport (Dōgo) Haneda Airport Air(JAL) Air(JAL) (all ports) (Dōgo) Itami Airport Dōzen (Dōgo) 1 hr 50 min Airport Bus Fast Ferry 30 min 50 min Bus Fast Ferry 30 min Izumo Airport 10 min Ferry 1 hr Air(JAC) Izumo Airport 10 min Ferry 1 hr Air(JAL) Air(JAC) Air(JAL) 30 min 1 hr 30 min 50 min 30 min Bus Matsue Bus Matsue Station Station 30 min 40 min Sakaiminato Port, Shichirui Port Bus Bus Air(ANA) Bus 30 min 30 min 40 min Sakaiminato Port, Shichirui Port Yonago Airport -

To Partner University of Shimane University
Shimane University(FY2016-2017) INTERNATIONAL PARTNER PROFILE SHIMANE UNIVERSITY §General Information Name of institution SHIMANE UNIVERSITY (国立大学法人 島根大学) Name of the head of the inst. Yasunao HATTORI, Ph.D. Title President Country / City JAPAN / Matsue and Izumo, Shimane Campus 2 campuses *Faculty of Medicine is located in Izumo Campus. URL http://www.shimane-u.ac.jp/ URL for international student http://kokusai.shimane-u.ac.jp/english/ exchange Responsible person of Akira DEGUCHI, Ph D. international exchange Director Center for International Exchanges Name of international office International Exchange Division Head of international office Kenichi TSUCHIYA (Mr.) Head International Exchange Division Contact for student exchange International Student Section of the International Exchange Division (Telephone) (+81-852-32-6106) (Fax) (+81-852-32-6481) (E-mail) ([email protected]) Contact for int’l agreement International Cooperation Section of the (Telephone) International Exchange Division (Fax) (+81-852-32-9735) (E-mail) (+81-852-32-6481) ([email protected]) Office hours 8:30 to 17:15 1 Shimane University(FY2016-2017) §Calendar Information Academic system Semester System *Semester 1: April – September - term exam: end of Jul. – beginning of Aug. - summer break: Aug. – Sep. *Semester 2: October –March - winter break: end of Dec. – beginning of Jan. - term exam: Feb. - spring break: Feb. – March Fiscal year From 1 April to 31 March Arrival dates for int’l exchange Semester 1: beginning of April student Semester 2: end of September Orientation for int’l students Semester 1: middle of April Semester 2: beginning of October §Academic Information Academic levels offered to Undergraduate, Master level, and PhD level. -

2016-2017 Unionpay International Global 100 Airport Duty Free Shops Campaign
2016-2017 UnionPay International Global 100 Airport Duty Free Shops Campaign Country/R # # Airport Duty Free Shop Promotion period Offer detail Location egion Arrival Hall, Level 5/Departure Hong Hong Kong From Jul 15, 2016 to Oct 15, 2016; from HKD200 off with a minimum purchase 1 1 1 DFS Hall, level 6, Terminal 1, Hong Kong International Airport Dec 15, 2016 to Feb 15, 2017 over HKD2,000 per single transaction Kong International Airport 1. NTD 1,200 off on one purchase of International Departure/Arrival From April 15, 2016 to May 31, 2016 Taipei Taoyuan NTD 30,000 or above area A, B, Terminal 1, 2 2 Everrich Duty Free From Aug 1, 2016 to Oct 31, 2016 International Airport 2. NTD 4,000 off on one purchase of International Departure/Arrival From Jan 1, 2017 to Feb 28, 2017 NTD 80,000 or above area C, Terminal 2 1. NTD 1,200 off on one purchase of From April 15, 2016 to May 31, 2016 Tasa Meng Duty Free NTD 30,000 or above International Departure/Arrival 3 3 Tasa Meng Duty Free From Aug 1, 2016 to Oct 31, 2016 Shop 2. NTD 4,000 off on one purchase of area D, Terminal 2 From Jan 1, 2017 to Feb 28, 2017 NTD 80,000 or above 1. NTD 1,200 off on one purchase of From April 15, 2016 to May 31, 2016 Taipei Songshan NTD 30,000 or above International Departure/Arrival 4 4 Everrich Duty Free From Aug 1, 2016 to Oct 31, 2016 Airport 2. -

ORIX Rent-A-Car Vehicle Drop-Off
ORIX Rent-A-Car http://car.orix.co.jp/eng/ Vehicle drop-off (one-way rental) is not available while JEP is in use. Telephone Shop Name Business hours (Reservation Center) (04/01~11/30)08:00~20:00 Aomori Ekimae Rental Site (12/01~03/31)08:00~19:00 Aomori Airport Rental Site 08:00~20:00 Hachinohe Ekimae Rental Site 08:00~20:00 Misawa Airport Rental Site 8:00~19:00 (04/01~11/30)08:00~20:00 Shin-Aomori Ekimae Ringo Rental Site (12/01~03/31)08:00~19:00 Morioka Ekimae Rental Site 08:00~20:00 Ichinoseki Ekimae Rental Site 8:00~20:00 Akita Airport Rental Site (04/01~03/31)08:00~20:00 (04/01~11/30)08:00~20:00 Akita Eki Nishi-guchi Rental Site (12/01~03/31)08:00~19:00 Odate Noshiro Airport Rental Counter (04/01~03/31)08:30~18:00 Sendai Airport Rental Site (04/01~03/31)08:00~20:00 Sendai Ekimae Rental Site (04/01~03/31)08:00~21:00 +81-3-6740-8775 Izumichuo Eki Nishi-guchi Rental Site (04/01~03/31)08:00~20:00 Sendai Eki Higashi-guchi Rental Site 08:00~20:00 Sendai Oroshimachi Rental Site 8:00~19:00 Yamagata Airport Rental Site (04/01~03/31)08:00~19:00 Shonai Airport Rental Site (04/01~03/31)08:00~19:00 Fukushima Eki Nishi-guchi Rental Site (04/01~03/31)08:00~20:00 Koriyama Eki Nishi-guchi Rental Site (04/01~03/31)08:00~20:00 (04/01~10/31)08:00~20:00 Hirosaki Ekimae Rental Site (11/01~03/31)08:00~19:00 Shin-Aomori Ekimae Hamanasu Rental Site (04/01~03/31)08:00~20:00 Hanamaki Airport Rental Site 09:00~19:00 Kitakami Ekimae Rental Site 08:00~20:00 Sendai Kokubuncho Rental Site (04/01~03/31)08:00~20:00 Furukawa Eki Higashi-guchi Rental Site 8:00~20:00 -

KOBE Biomedical Innovation Cluster
Start in Kobe. Innovate in Kobe. KOBE Biomedical Innovation Cluster Business Launch Support Guide Foundation for KOBE Biomedical Innovation Cluster Biomedical Research and Innovation at Kobe (FBRI) The City of Kobe launched the Kobe Biomedical Innovation Cluster (KBIC) as a recovery project to rebuild the As a core support organization of Kobe Biomedical Innovation Center, FBRI aims to create economy of Kobe, which was severely damaged by the Great Hanshin-Awaji Earthquake that occurred on January 17, groundbreaking medical technologies and innovations by promoting collaboration and integration 1995. Since 1998, the City of Kobe has developed an R&D hub for advanced medical technology on Port Island, an among industries, universities and the governmental and life science sectors, to become Asia’s artificial island, and through collaboration among industries, universities and the governmental and medical sectors, it leading biomedical cluster. Dr. Tasuku Honjo, President has built up a cluster of medical-related industries that will be the growth engine for the life sciences industry in the 21st century. To date, it has grown to become Japan's largest biomedical cluster with a concentration of about 370 Organizations cutting-edge research institutes, highly specialized hospitals, medical-related companies and universities. Institute of Biomedical Translational Research Research and Center for Cluster Research and Center for Medical Development Center Development and Innovation(IBRI) Innovation(TRI) for Cell Therapy (RDC) Coordination (CCD) Creation of new medical seeds with Support for the development of Global standardization of regenera- Providing one-stop services to respond a focus on "immunology R&D," new medical seeds in Japan and tive medicine from Kobe through to a wide range of support needs in a "R&D, for the aging" and "regenera- overseas, with a focus on the the development of a next-genera- centralized manner, from the identifica- tive medicine R&D” research and development seeds tion cell cultivation systems, etc. -

Danh Sách Các Phòng Chờ Loungekey™ & Các Phòng
DANH SÁCH CÁC PHÒNG CHỜ LOUNGEKEY™ & CÁC PHÒNG CHỜ TẠI NHẬT BẢN, HAWAII Status/ Lounges/ Country/ Service/ STT Address/ Địa Chỉ Airport/ Sân Bay Trạng Thái Phòng Chờ Quốc Gia Dịch Vụ No. 77 China Shanghai Pudong 1 On-going Eastern Plaza Terminal 2 China LoungeKey International Airport Premium Lounge Plaza Premium Taoyuan International 2 On-going Terminal 1, Zone D Taiwan LoungeKey Lounge Airport Plaza Premium Taoyuan International 3 On-going Terminal 2, Zone A Taiwan LoungeKey Lounge Airport Terminal 1, Airside, East SKY HUB Lounge Incheon International 4 On-going Wing, 4th Floor, opposite Korea LoungeKey (East) Airport Gate 11 Terminal 1, Airside, SKY HUB Lounge Incheon International 5 On-going West Wing, 4th Floor, Korea LoungeKey (West) Airport opposite Gate 43 Matina Lounge Terminal 1, Airside, East Incheon International 6 On-going Korea LoungeKey (East) Wing, 4th Floor Airport Matina Lounge Terminal 1, Airside, Incheon International 7 On-going Korea LoungeKey (West) West Wing, 4th Floor Airport Plaza Premium International Departures, Kuala Lumpur 8 On-going Malaysia LoungeKey Lounge Satellite Terminal International Airport Plaza Premium Singapore Changi 9 On-going Terminal 1 Singapore LoungeKey Lounge Airport Singapore Changi 10 On-going The Green Market Terminal 2 Singapore LoungeKey Airport Blossom - Premium Singapore Changi 11 On-going Terminal 4 Singapore LoungeKey Lounge Airport Miracle First Class International Concourse Suvarnabhumi 12 On-going Thailand LoungeKey Lounge A International Airport 1 Status/ Lounges/ Country/ Service/ -

Full Automation of Aeronautical Meteorological Observations and Reports at Aerodromes
Full automation of aeronautical meteorological observations and reports at aerodromes August 2016 Japan Meteorological Agency Blank page Amendments and corrigenda Date of issue Detail 10 February 2017 First edition (body part only) issued 6 March 2017 Attachments and annexes added (provisional; to be revised after proofread- ing) along with editorial amendments 29 March 2017 Revised based on proofreading 19 March 2019 Addition of aerodromes with full automation 30 March 2020 Addition of aerodromes with full automation 8 October 2020 Subtraction of an aerodrome due to suspension of aviation weather service 2 September 2021 Addition of aerodromes with full automation (i) Blank page (ii) CONTENTS Full automation of aeronautical meteorological observations and reports at aerodromes 1 Introduction .................................................................................................................................. 1 2 Plan for full automation ............................................................................................................... 1 3 Automated observations and reports ......................................................................................... 2 4 Characteristics of values to be reported by automated METAR/SPECI ................................ 4 5 Differences between automated reports (automated METAR/SPECI) and manned reports (manned METAR/SPECI and SCAN) ................................................. 5 6 Differences between automated METAR/SPECI and conventional METAR AUTO ........... -

KODY LOTNISK ICAO Niniejsze Zestawienie Zawiera 8372 Kody Lotnisk
KODY LOTNISK ICAO Niniejsze zestawienie zawiera 8372 kody lotnisk. Zestawienie uszeregowano: Kod ICAO = Nazwa portu lotniczego = Lokalizacja portu lotniczego AGAF=Afutara Airport=Afutara AGAR=Ulawa Airport=Arona, Ulawa Island AGAT=Uru Harbour=Atoifi, Malaita AGBA=Barakoma Airport=Barakoma AGBT=Batuna Airport=Batuna AGEV=Geva Airport=Geva AGGA=Auki Airport=Auki AGGB=Bellona/Anua Airport=Bellona/Anua AGGC=Choiseul Bay Airport=Choiseul Bay, Taro Island AGGD=Mbambanakira Airport=Mbambanakira AGGE=Balalae Airport=Shortland Island AGGF=Fera/Maringe Airport=Fera Island, Santa Isabel Island AGGG=Honiara FIR=Honiara, Guadalcanal AGGH=Honiara International Airport=Honiara, Guadalcanal AGGI=Babanakira Airport=Babanakira AGGJ=Avu Avu Airport=Avu Avu AGGK=Kirakira Airport=Kirakira AGGL=Santa Cruz/Graciosa Bay/Luova Airport=Santa Cruz/Graciosa Bay/Luova, Santa Cruz Island AGGM=Munda Airport=Munda, New Georgia Island AGGN=Nusatupe Airport=Gizo Island AGGO=Mono Airport=Mono Island AGGP=Marau Sound Airport=Marau Sound AGGQ=Ontong Java Airport=Ontong Java AGGR=Rennell/Tingoa Airport=Rennell/Tingoa, Rennell Island AGGS=Seghe Airport=Seghe AGGT=Santa Anna Airport=Santa Anna AGGU=Marau Airport=Marau AGGV=Suavanao Airport=Suavanao AGGY=Yandina Airport=Yandina AGIN=Isuna Heliport=Isuna AGKG=Kaghau Airport=Kaghau AGKU=Kukudu Airport=Kukudu AGOK=Gatokae Aerodrome=Gatokae AGRC=Ringi Cove Airport=Ringi Cove AGRM=Ramata Airport=Ramata ANYN=Nauru International Airport=Yaren (ICAO code formerly ANAU) AYBK=Buka Airport=Buka AYCH=Chimbu Airport=Kundiawa AYDU=Daru Airport=Daru