Guadeloupe Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Information on the Chemical. Physical and Biological
93 NEW INFORMATION ON THE CHEMICAL. PHYSICAL AND BIOLOGICAL PROPERTIES OF DRY BEANS LOUIS B. ROCKLAND, EUFROCINA M. ZARAGOSA AND DULCIE M. HAHN Western Regional Research Laboratory, Agricultural Research Service, U. S. Department of Agriculture, Berkeley, California 94710 INTRODUCTION Basic studies on the physical, chemical and biological properties of dry beans during the past 15 years (1-5, 8, 12) have been indispensable to the evolution of a new process for preparing dry beans as human food; and the generation of a series of more acceptable products from commercial varieties of legumes (6, 7, 9-11). One dividend of the basic work has been the accumulation of new knowledge relevant to a variety of problems concerned with bean processing. Chemical studies on whole dry, raw water-soaked,and processed quick-cooking beans, as well as their cooked counterparts, established a rational basis for improving bean processing methods and at the same time provided data on bean nutrients and nutritional properties. The latter data will be useful in preparing to comply with new FDA recommendations for nutritional labeling. Visual and scanning electron microscopy have been employed to determine the influences of soaking, processing and cooking on the morphology of cells in large Lima bean cotyledons. Critical changes in cotyledon proteins, during processing and cooking, have been characterized by polyacrylamide gel electrophoresis. Electrophoretic characterization of bean proteins promises to provide a completely new basis for distinguishing closely related legume varieties. Only a fraction of a single seed may be used to distinguish hybrid strains and thereby conceivably minimize the need for seasonal agronomic evaluations. -

Lima Bean (Phaseolus Lunatus L.) – a Health Perspective
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 9, ISSUE 02, FEBRUARY 2020 ISSN 2277-8616 Lima Bean (Phaseolus Lunatus L.) – A Health Perspective Lourembam Chanu Bonita, G. A. Shantibala Devi and Ch. Brajakishor Singh Abstract: Lima beans are underutilized crops with a good nutritional profile. They are very good sources of proteins, minerals, dietary fibres and the essential amino acid lysine which is lacking in cereals. They are also good sources of bioactive compounds. Some of these compounds can adversely affect the health of the consumers, and are known as antinutritional compounds. Compounds like phytates, saponins, and phenols reduce the bioavailability of minerals. Cyanogenic glycosides which can be hydrolyzed into the highly toxic hydrogen cyanide are also known to be present in lima beans. Its content is highly variable among the different varieties. Another reason which makes lima beans unpopular to consumers is the presence of flatulence causing oligosaccharides. Different methods have been proposed to remove the content of these undesirable components. For most of them, soaking and cooking are enough to reduce their content to a desirable level. Bioactive compounds, including those which have been traditionally designated as antinutritional factors, can also affect the health in a beneficial way. Many compounds present in lima beans such as phenolic compounds are known to be antioxidants. The health promoting effects of lima beans and its constituents reported in literature so far include hypoglycemic, anti-HIV, anticancer, antihypertensive, bile acid binding, gastroprotective, protection from cardiovascular diseases and antimicrobial properties. These nutraceutical properties of lima beans are discussed in this review. Index Terms: Lima bean, nutritional, antinutritional, health, bioactive, anti-HIV, anticancer —————————— —————————— 1. -

Effects of Feeding Spodoptera Littoralis on Lima Bean Leaves. I
Effects of Feeding Spodoptera littoralis on Lima Bean Leaves. I. Membrane Potentials, Intracellular Calcium Variations, Oral Secretions, and Regurgitate Components1 Massimo Maffei, Simone Bossi, Dieter Spiteller, Axel Mitho¨fer, and Wilhelm Boland* Department of Plant Biology, University of Turin, Turin, Italy (M.M., S.B.); and Max Planck Institute for Chemical Ecology, D–07745 Jena, Germany (D.S., A.M., W.B.) Membrane potentials (Vm) and intracellular calcium variations were studied in Lima bean (Phaseolus lunatus) leaves when the Mediterranean climbing cutworm (Spodoptera littoralis) was attacking the plants. In addition to the effect of the feeding insect the impact of several N-acyl Glns (volicitin, N-palmitoyl-Gln, N-linolenoyl-Gln) from the larval oral secretion was studied. The results showed that the early events upon herbivore attack were: a) a strong Vm depolarization at the bite zone and an isotropic wave of Vm depolarization spreading throughout the entire attacked leaf; b) a Vm depolarization observed for the regurgitant but not with volicitin {N-(17-hydroxy-linolenoyl)-Gln} alone; c) an enhanced influx of Ca21 at the very edge of the bite, which is halved, if the Ca21 channel blocker Verapamil is used. Furthermore, the dose-dependence effects of N-acyl Gln conjugates- triggered influx of Ca21 studied in transgenic aequorin-expressing soybean (Glycine max) cells, showed: a) a concentration- dependent influx of Ca21; b) a configuration-independent effect concerning the stereochemistry of the amino acid moiety; c) a slightly reduced influx of Ca21 after modification of the fatty acid backbone by functionalization with oxygen and; d) a comparable effect with the detergent SDS. -

10-Step Guide to Saving Seeds and Avoiding Cross-Pollination
10-Step Guide to Saving Seeds and Avoiding Cross-Pollination Christy Marsden, Horticulture Educator with U of MN Extension 1. Know your plant Open pollination occurs when pollen can flow freely between cultivars of the same species. Look for (OP) next to the plant name. Hybrid plants are borne from intentional cross-pollination. Seeds from hybrid plants will produce plants that differ from the parent plants. Look for (F1) next to the plant name. Cross pollination occurs when pollen is shared between to plants of the same species but different cultivars or varieties. Resulting seed won’t produce true-to-type plants, so it’s important to prevent cross-pollination from occurring. The scientific name of each plant [read as Genus species ‘cultivar/variety’] will tell you the potential for cross-pollination. For example, a Valentine bean (Phaseolus vulgaris ‘Valentine’) will cross with a Dappled Grey bean (Phaseolus vulgaris ‘Dappled Grey’) because they are the same species. Neither will cross with a Phebe Vinson lima bean (Phaseolus coccineus ‘Phebe Vinson’) because they are different species. 2. Know the lifecycle Annual plants will complete their life cycle in one growing season, producing seed in one year. Biennial plants need two growing seasons, typically with an overwintering period, to produce seed. In the Midwest, biennial plants must be overwintered in root cellars to avoid winter cold damage. Common annuals: arugula, basil, beans, some broccoli, cilantro, corn, cucumber, dill, eggplant, lettuce, melon, some mustard greens, okra, pea, pepper, pumpkin, radish, spinach, squash, tomato, watermelon Common biennials: beet, some broccoli, Brussels sprouts, cabbage, cauliflower, celery, celeriac, Swiss chard, chicory, collards, kale, leeks, some mustard greens, onions, parsley, parsnip, radicchio, rutabaga, turnip 3. -

The Solanaceae
PHASEOLUS LESSON TWO The Genus PHASEOLUS and How to Grow Beans In this lesson we will continue our study of the LEGUME family by concentrating on the GENUS Phaseolus, We will study some of the different SPECIES in the GENUS Phaseolus. We will also study about growing beans. For this lesson to be complete you must: ___________ do everything in bold print; ___________ answer the questions at the end of the lesson; ___________ learn to identify the different beans (use the study materials at www.geauga4h.org); and ___________ complete one of the projects at the end of the lesson. Parts of the lesson are underlined and/or in a different print. These sections include reference to past Plant Masters projects. Try your best to answer using your old project books or your memory. Younger members and new members can ignore these sections. Parts of the lesson are underlined. Younger members can ignore these parts. WORDS PRINTED IN ALL CAPITAL LETTERS may be new vocabulary words. For help, see the glossary at the end of the lesson. INTRODUCTION TO THE Phaseolus Phaseolus - Bean Group The word “bean” is used for plants and FRUITS of many different GENERA. Originally it was used for the broad bean, or Vicia faba. However, when other bean-type FRUITS came from other parts of the world, the word was extended to the plants in several other GENERA. It is even used for the FRUITS of plants which are not “beans” at all. However, the FRUITS of these plants resemble beans, for example coffee beans, cocoa beans, and vanilla beans. -

BUTTERY DELIGHT Lima Bean Lima Beans Are a Good Source of Protein
BUTTERY DELIGHT lima bean Lima beans are a good source of protein. When they are combined with a whole grain such as brown rice, they provide protein comparable to that of meat or dairy foods. Lima beans, like other legumes, are packed with fiber. Just one cup will meet about half of your daily fiber needs. DID YOU KNOW? SHOPPING, PREPARING MARINATED THREE- serves 4 AND STORING BEAN SALAD Lima beans are sometimes called “butter beans” Fresh lima beans should be because of their starchy, yet stored whole, in their pods, in • 1 cup lima beans, cooked buttery texture. the refrigerator crisper where • 1 cup green beans, cooked Because lima beans are they can keep fresh for a few • 1 cup red kidney beans, packed with protein, B days. Frozen lima beans do cooked vitamins and iron, they are a not need to be thawed before • 1 medium onion, thinly sliced particularly great choice for being cooked. • 1/2 cup green bell pepper, chopped vegetarians. You can find lima beans fresh, • 1 cup fat-free Italian salad dressing The baby lima beans have dried, canned or frozen. a creamier, more delicate 1. In a large bowl, combine the lima beans, green beans, kidney beans, onion taste than the larger, COOKING TIPS starchier lima beans. and green bell pepper. 2. Pour the dressing over the vegetables and Succotash is a well-known If cooking dried lima beans, avoid adding salt or acidic toss lightly. Native American dish that 3. Cover the bowl and marinate in the ingredients, such as vinegar, combines lima beans with refrigerator for at least one hour. -

Effect of Different Treatments on Nutritional Value of Lima Bean (Phaseolus Lunatus) and Its Utilization in Biscuit Manufacture
Food and Nutrition Sciences, 2021, 12, 372-391 https://www.scirp.org/journal/fns ISSN Online: 2157-9458 ISSN Print: 2157-944X Effect of Different Treatments on Nutritional Value of Lima Bean (Phaseolus lunatus) and Its Utilization in Biscuit Manufacture Sahar S. El-Gohery Bread and Pasta Department, Food Technology Research Institute, Agricultural Research Center, Giza, Egypt How to cite this paper: El-Gohery, S.S. Abstract (2021) Effect of Different Treatments on Nutritional Value of Lima Bean (Phaseolus Physical properties, chemical composition, minerals content, amino acids lunatus) and Its Utilization in Biscuit Man- profile and anti-nutritional factors i.e. phytic acid, tannins and trypsin inhi- ufacture. Food and Nutrition Sciences, 12, bitor of lima bean seeds (Phaseolus lunatus) which newly cultivated in Egypt 372-391. https://doi.org/10.4236/fns.2021.124029 were investigated. Addition to study the effect of common processing me- thods (soaking, cooking, roasting, and dehulling) on the chemical composi- Received: March 8, 2021 tion, minerals content and anti-nutritional factors of lima bean seeds in order Accepted: April 19, 2021 to be used in biscuit preparation. Results showed that protein content was Published: April 22, 2021 significantly (P ≤ 0.05) increased in dehulled Lima bean (27.06%) compared Copyright © 2021 by author(s) and to raw lima bean (26.02%). All processing methods significantly (P ≤ 0.05) Scientific Research Publishing Inc. increased Soluble Dietary Fiber (SDF), however soaked seeds contained the This work is licensed under the Creative highest significant Total Dietary Fiber (TDF) (30.18%) and Insoluble Dietary Commons Attribution International License (CC BY 4.0). -

Phenotypic Diversity in Lima Bean Landraces Cultivated in Brazil, Using
Phenotypic diversity in lima bean landraces cultivated in Brazil, using the Ward-MLM strategy Raimundo Nonato Oliveira Silva1*, Marília Lobo Burle2, Juliano Gomes Pádua2, Ângela Celis de Almeida Lopes3, Regina Lucia Ferreira Gomes3, and Jaime Martínez-Castillo4 ABSTRACT INTRODUCTION Lima bean (Phaseolus lunatus L.) is an important source Lima bean (Phaseolus lunatus L.) is composed of two botanical of protein for people as it contributes all of the essential varieties: var. silvester for the wild material and var. lunatus for amino acids necessary for humans. In Brazil, lima bean the domesticated one (Baudet, 1977). Studies have indicated has a great relevance, mainly in the Northeast, where the existence of three major gene pools: One Andean gene pool it is an alternative income in addition to a food source. (A) and two Mesoamerican gene pools (MI and MII); with It has a high degree of phenotypic variation for seed one domestication event in each of them: 1) for A, the mid- traits, which are important for understanding the genetic altitude western valleys between Ecuador and Peru in South diversity and origin of this crop. We aimed to characterize America (Maquet et al., 1997); 2) the central eastern region 166 accessions of cultivated lima bean from Brazil using from Mexico for MI (Serrano-Serrano et al., 2012; Andueza- qualitative and quantitative descriptors through the Ward- Noh et al., 2013); and 3) the region located between Guatemala MLM (Modified Location Model) in order to analize the and Costa Rica for MII (Andueza-Noh et al., 2013; 2015). Recent organization of the genetic diversity and the origin of this evidence from microsatellite markers have suggested another germplasm. -
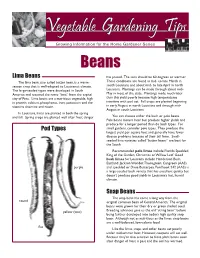
Lima Beans Snap Beans Pod Types
Beans Lima Beans has passed. The soils should be 60 degrees or warmer. The lima bean, also called butter bean, is a warm- These conditions are found in mid- to late March in season crop that is well-adapted to Louisiana’s climate. south Louisiana and about mid- to late April in north The large-seeded types were developed in South Louisiana. Plantings can be made through about mid- America and received the name “lima” from the capital May in most of the state. Plantings made much later city of Peru. Lima beans are a nutritious vegetable, high than this yield poorly because high temperatures in protein, calcium, phosphorus, iron, potassium and the interfere with pod set. Fall crops are planted beginning vitamins thiamine and niacin. in early August in north Louisiana and through mid- August in south Louisiana. In Louisiana, limas are planted in both the spring and fall. Spring crops are planted well after frost danger You can choose either the bush or pole beans. Pole beans mature later but produce higher yields and produce for a longer period than do bush types. For Pod Types small gardens, consider pole types. They produce the largest yield per square foot and generally have fewer disease problems because of their tall form. Small- seeded lima varieties called “butter beans” are best for the South. Recommended pole limas include Florida Speckled, King of the Garden, Christmas or Willow Leaf. Good bush limas for Louisiana include Henderson Bush, Eastland, Jackson Wonder, Thorogreen, Cangreen (AAS) purple and speckled or Dixie Butterpea. Fordhook 242 (AAS) is a large-seeded bush variety that has excellent quality but doesn’t produce good yields in Louisiana’s hot, humid climate. -

Successful Lima Bean Production
Successful Lima Bean Production Lima beans (Phaseolus lunatus L.) are an important crop in Delaware and the mid- Atlantic region of the United States. Grown primarily for processing, lima beans are planted on more acres in Delaware than any other vegetable crop. Other major production areas for succulent, green lima beans grown for canning or freezing are California, the Pacific Northwest, and New York State. Delaware and nearby Marlyan plants more acreage annually (20,000 acres) than any of these areas. The total annual acreage of lima beans planted for succulent bean processing purposes in the United States is approximately 40,000 acres. Lima beans are also planted in California and other western areas for dry bean use. In Delaware, lima beans are considered to be a cornerstone crop of the vegetable- processing industry. Because the state is one of the few areas that produce the crop, Delaware offers a unique item for local processors to sell. Lima beans are double-cropped on as much as three-fourths of the acreage, thus offering producers maximum utilization of land. Limas are often planted in June or July after a pea or small grain crop. The same harvesting equipment is used for peas (Pisum sativum L.) and lima beans, so. capitalization costs can be spread over two crops per year. Typically, the grower contracts with a processing company for a certain acreage. In most cases, the processing company performs the harvest and raw product delivery functions, although there are instances of growers owning their own harvest equipment. In the latter case, the processor pays a higher price for the lima beans. -

Asian Soybean Rust
Agriculture and Natural Resources Asian Soybean Rust Cliff Coker Introduction Since 1902, P. pachyrhizi has Associate Professor become a problem in various parts of Asian soybean rust is a serious Asia and Australasia. Kim Hurst disease threat to Arkansas and U.S. During the 1990s, the disease was Program Associate soybean production. On November 10, 2004, the disease was confirmed in reported in Africa, spreading to two soybean fields in Louisiana and various soybean-producing countries Terry Kirkpatrick shortly thereafter in Mississippi, there. Professor Florida, Georgia, Alabama, Arkansas, Missouri, South Carolina and In 2000-2001, P. pachyrhizi was Tennessee. The disease was found on John Rupe detected in Paraguay, South America, soybean plants that remained green and confirmed in Brazil and Professor very late in the season due to various Argentina in 2002 and Bolivia in factors. It was also noted on kudzu in 2003. It has caused major problems in Florida. All available evidence Chris Tingle these countries since its introduction. suggests that the disease was Extension Agronomist - introduced by windborne spores Soybeans carried from the northern edge of In 2004, the disease was South America to the southern U.S. by confirmed in Colombia, South Mark Trent Hurricane Ivan in September. On America, north of the equator, making its introduction into the southern U.S. Program Associate February 23, 2005, Asian soybean rust was confirmed as surviving on kudzu imminent – and this later occurred in in Pasco County, Florida, indicating September via Hurricane Ivan. successful overwintering. Reasons for the rapid geographic spread of Asian soybean rust from This fact sheet is intended to Asia to Africa and the Western provide information on biology, Hemisphere during the past decade identification and control of are not well understood. -

Insect Pests of Beans & Peas in New England
Insect Pests of Beans & Peas in New England Alan Eaton University of New Hampshire Cooperative Extension 252 Spaulding Hall, UNH, Durham NH 03824 My comments here are heavily influenced by my experiences in New Hampshire, but I’ve included information from southern New England as well. To me, the main message is that insect problems aren’t common here on beans and peas. I have listed them in order of significance: Potato leafhopper is a sporadic pest. It doesn’t overwinter in New England. It re- invades New England every summer, by being blown in from the south. We usually find our first ones (adults of course) in June, sometimes July. They are yellow-green in color, and quickly fly when disturbed. In most situations, any yellow-green leafhopper you find on beans in summer is probably this one. You can confirm identification by the fact that the leafhoppers have a series of white lines on and just behind the top of the head. You’ll need slight magnification to see this. This insect has piercing-sucking mouthparts, and it injects toxic saliva when it feeds. Leaves that have been attacked quickly turn yellow at the edges. The yellow spreads and eventually the leaf edges die. In some years this is a problem, but others it is a no-show in New Hampshire. I’ve never seen damage on peas, but I have on bean, cantaloupe, potato, eggplant, alfalfa, basil, raspberry, apple, and dahlia. If they number more than one per leaflet on bean (yes, you should count), it may be helpful to apply an insecticide.