Metabolic Profiling, in Vitro Propagation, and Genetic Assessment of the Endangered Rare Plant Anarrhinum Pubescens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Does Phloem Physiology Affect Plant Ecology?
Plant, Cell & Environment (2016) 39,709–725 doi: 10.1111/pce.12602 Review Allocation, stress tolerance and carbon transport in plants: how does phloem physiology affect plant ecology? Jessica A. Savage1, Michael J. Clearwater2, Dustin F. Haines3, Tamir Klein4, Maurizio Mencuccini5,6, Sanna Sevanto7, Robert Turgeon8 &CankuiZhang9 1Arnold Arboretum of Harvard University, 1300 Centre Street, Boston, MA 02131, USA, 2School of Science, University of Waikato, Hamilton 3240, New Zealand, 3Department of Environmental Conservation, University of Massachusetts, 160 Holdsworth Way, Amherst, MA 01003, USA, 4Institute of Botany, University of Basel, Schoenbeinstrasse 6, 4056 Basel, Switzerland, 5School of GeoSciences, University of Edinburgh, Crew Building, West Mains RoadEH9 3JN Edinburgh, UK, 6ICREA at CREAF, Campus de UAB, Cerdanyola del Valles, Barcelona 08023, Spain, 7Earth and Environmental Sciences Division, Los Alamos National Laboratory, Los Alamos, NM 87545, USA, 8Plant Biology Section, School of Integrative Plant Science, Cornell University, Ithaca, NY 14853, USA and 9Department of Agronomy, Purdue University, West Lafayette, IN 47907, USA ABSTRACT potentially influencing everything from growth and allocation to defense and reproduction (Fig. 1). The phloem’srolein Despite the crucial role of carbon transport in whole plant shaping many ecological processes has intrigued scientists for physiology and its impact on plant–environment interactions decades, but proving a direct connection between phloem and ecosystem function, relatively little research has tried to ex- physiology and plant ecology remains challenging. amine how phloem physiology impacts plant ecology. In this re- Carbon transport occurs in a series of stacked cells, sieve el- view, we highlight several areas of active research where ements, which in angiosperms form long continuous conduits inquiry into phloem physiology has increased our understand- called sieve tubes (for details on cell ultrastructure, see Froelich ing of whole plant function and ecological processes. -
2007 Catalog
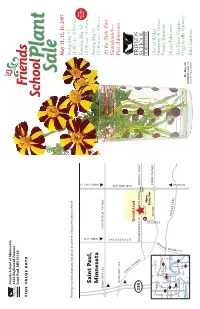
Friends School of Minnesota 1365 Englewood Avenue Saint Paul, MN 55104 TIME VALUE DATA May 11, 12, 13, 2007 Friday,May 11 If you have received a duplicate copy, please let us know, and pass the extra to a friend! 11:00 A.M.–8:00 P.M. New Saturday Saturday,May 12 Hours Saint Paul, 10:00 A.M.–6:00 P.M. Sunday,May 13 FROM 35W Minnesota FROM HWY 36 12:00 NOON–4:00 P.M. FROM HWY 280 LARPENTEUR AVENUE At the State Fair Grandstand— FROM HWY 280 Free Admission C O M O CLEVELAND AVE A SNELLING AVE V E Grandstand N U 280 E COMMONWEALTH DAN PATCH Main MIDWAY PKWY Gate P Minn. State Fair 94 Coliseum COMO AVENUE 35W White Shoreview Glacial Ridge Brooklyn Ctr Bear Lake 694 35E E U CANFIELD Growers: A Green Plymouth Crystal 94 Roseville N 36 E 494 Snelling Ave. 694 V 169 Saint Paul Family Business 280 A 394 35E 100 94 D Minnetonka Minneapolis E N N Woodbury ERGY Hosta Takeover! O P Edina 494 ARK 62 M Richfield Y 61 Eden 494 Prairie A Are These Veggies 35W Inver Grove R Heights Bloomington Eagan FROM 94 Organically Grown? 52 Mr. Majestic Shakopee 35E Burnsville marigold, page 12 Photo by Nancy Scherer Bird Gardens 18th Annual Friends School Plant Sale May 11, 12 and 13, 2007 Friday 11:00 A.M.–8:00 P.M.• Saturday 10:00 A.M.–6:00 P.M. Sunday 12:00 NOON–4:00 P.M.Sunday is half-price day at the Minnesota State Fair Grandstand Friends School of Minnesota Thank you for supporting Friends School of Minnesota by purchasing plants at our sale. -

Technical Report Series No. 287 Advisory List of Environmental Weeds in Victoria
Advisory list of environmental weeds in Victoria M. White, D. Cheal, G.W. Carr, R. Adair, K. Blood and D. Meagher April 2018 Arthur Rylah Institute for Environmental Research Technical Report Series No. 287 Arthur Rylah Institute for Environmental Research Department of Environment, Land, Water and Planning PO Box 137 Heidelberg, Victoria 3084 Phone (03) 9450 8600 Website: www.ari.vic.gov.au Citation: White, M., Cheal, D., Carr, G. W., Adair, R., Blood, K. and Meagher, D. (2018). Advisory list of environmental weeds in Victoria. Arthur Rylah Institute for Environmental Research Technical Report Series No. 287. Department of Environment, Land, Water and Planning, Heidelberg, Victoria. Front cover photo: Ixia species such as I. maculata (Yellow Ixia) have escaped from gardens and are spreading in natural areas. (Photo: Kate Blood) © The State of Victoria Department of Environment, Land, Water and Planning 2018 This work is licensed under a Creative Commons Attribution 3.0 Australia licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo, the Department of Environment, Land, Water and Planning logo and the Arthur Rylah Institute logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Printed by Melbourne Polytechnic, Preston Victoria ISSN 1835-3827 (print) ISSN 1835-3835 (pdf)) ISBN 978-1-76077-000-6 (print) ISBN 978-1-76077-001-3 (pdf/online) Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. -

Arboretumplants.Org
Plant # Scientific Name Common name Flower Time “ High Spring Affair Plant List SUN 166 CALLIRHOE ALCAEOIDES 'LOGAN CALHOUN' POPPY MALLOW WHITE APR-OCT 8-12 SUN 167 CALLIRHOE DIGITATA POPPY MALLOW RED MAY-SEP 24-48 SUN 168 CALLIRHOE INVOLUCRATA POPPY MALLOW PINK JUN-AUG 6-12 Order ONLINE by April 19 SUN 169 CEANOTHUS AMERICANUS NEW JERSEY TEA WHITE MAY-JUL 36-48 Select pickup time slot at Lincoln’s Lancaster Event Center SUN 170 CENTRANTHUS RUBER 'PRETTY BETSY' RED VALERIAN RED MAY-JUN 18-36 Prices are listed individually online; most are $3.50. Join Nebraska SUN 171 CEPHALANTHUS OCCIDENTALIS BUTTONBUSH WHITE JUN 60-96 Statewide Arboretum for 10% discount on Spring Affair plants and SUN 172 CERASTIUM TOMENTOSUM SNOW IN SUMMER WHITE JUN 6-12 15% discount on Arboretum plants year-round. SUN 173 CHRYSANTHEMUM 'CLARA CURTIS' MUM PINK AUG-SEP 14 SUN 174 CHRYSANTHEMUM 'MARY STOKER' MUM YELLOW AUG-SEP 24-30 SUN 175 CHRYSANTHEMUM WEYRICHII 'PINK BOMB' MUM PINK AUG-SEP 6-12 arboretumplants.org SUN 176 CLEMATIS ALPINA 'BETINA' CLEMATIS BLUE APR-SEP 96-108 SUN 177 CLEMATIS 'ARABELLA'' CLEMATIS PURPLE MAY-JUL 48-96 Plant # Scientific Name Common name Flower Time “ High SUN 178 CLEMATIS 'BLUE EXPLOSION' CLEMATIS BLUE JUL-SEP 72-96 PERENNIALS for SUN SUN 179 CLEMATIS 'GYPSY QUEEN' CLEMATIS PINK MAY-JUL 72-96 SUN 180 CLEMATIS 'HENRYI' CLEMATIS PURPLE JUN-SEP 96 SUN 100 ACANTHUS MOLLIS BEAR’S BREECHES WHITE JUN-JUL 36-60 SUN 181 CLEMATIS HEX. 'MONGOLIAN SNOWFLAKES' CLEMATIS WHITE JUN-OCT 36-48 SUN 101 ACANTHUS SPINOSUS BEAR’S BREECHES MAUVE JUN-AUG 36-48 -

Lianas and Climbing Plants of the Neotropics: Plantaginaceae
GUIDE TO THE GENERA OF LIANAS AND CLIMBING PLANTS IN THE NEOTROPICS PLANTAGINACEAE By Mark T. Strong (Jan 2021) A widely distributed family of primarily herbs, subshrubs or shrubs with about 100 genera and ca. 1,900 species worldwide. In the Neotropics, they are represented by about 45 genera and ca. 400 species. Five genera and 14 species are treated below as climbing plants. These occur in a diversity of habitats from desert scrub to montane cloud forests. Diagnostics: In vegetative condition, climbing Plantaginaceae have stems that are quadrangular or terete; leaves are opposite, alternate or sometimes verticillate, glabrous or glandular- pubescent, simple, and stipules are absent. In the order Lamiales, the quadrangular stems and simple opposite leaves of some Plantaginaceae Russelia syringifolia Schltdl. & Cham., photo by J. might be confused with Acanthaceae, Amith Gesneriaceae, Lamiaceae and Verbenaceae. Acanthaceae generally have ovaries with hook-like placental tissue and capsules with explosive dehiscence while in Gesneriaceae, ovaries are unilocular with parietal placentation. Lamiaceae and Verbenaceae have 2-ovulate ovaries and the fruit is a 4-parted schizocarp or dry indehiscent drupe. GENERAL CHARACTERS 1. STEMS. Quadrangular (sometimes winged) or terete in cross section, commonly solid, but hollow in some species of Russelia (e.g. R. campechiana Standl.; fig. 1a), xylem with deep phloem wedges in species of Russelia (e.g., R. contrerasii B.L. Turner; fig. 1b). Vessels narrow and commonly radially disposed (Metcalfe & Chalk, 1957). No visible exudates reported for the group. 2. PUBESCENCE. Glabrous or glandular-pubescent. 3. LEAVES. Alternate, opposite, verticillate or opposite proximally and alternate distally, the blades deltoid to cordiform, hastate or sagittate at base with palmate venation, or sometimes linear to lanceolate or ovate-lanceolate with pinnate venation, entire or with dentate margins, glabrous or glandular-pubescent; stipules absent. -

Second Issue of the Mediterranean Garden
THE Mediterranean Garden No. 2 Autumn 1995 THE MEDITERRANEAN GARDEN THE MEDITERRANEAN GARDEN A journal for gardeners in all the mediterranean climate regions of the world Published by the Mediterranean Garden Society, PO Box 14, Peania GR-19002, Greece. www.MediterraneanGardenSociety.org i Editors Caroline Harbouri Derek Toms Translations Graziella Seferiades Caroline Harbouri Illustrations Derek Toms * * * The Mediterranean Garden Society is a non-profit-making association which acts as a forum for everyone who has a special interest in the plants and gardens of the region. For details, please contact The Secretary, MGS, PO Box 14, Peania, 19002 Greece. Phototypeset by Eikonotypo S.A. Elia Eliou 64 & Koutsonika 5 Neos Kosmos 117 44 Athens Printed on recycled paper by Corfu Graphics Smolenski 9 & Telemachou 15 Neapolis 114 72 Athens Copyright of all articles remains with the authors. Views expressed by contributors are not necessarily those of the editors or of the Mediterranean Garden Society. ISSN 1106-5826 ii CONTENTS Meditorial 1 Domaine du Prieuré Joanna Millar 6 Inspired by Beth Chatto’s Garden Caroline Harbouri 12 Propagating Australian Plants Jeff Irons 16 Easy Flowering Plants John Calderwood 19 Kankerbos Tom Wellsted 21 Agapanthus for Your Garden Trevor Nottle 22 Acclimatisation Problems Piero Caneti 26 Bring the Plants of the Mountain into Your Garden Argyroupolis Environment Group 29 The Historic Gardens Foundation Gillian Mawrey 34 The Day Trip Russell Read 36 The Garden in Autumn Jenny Bussey 42 Sundries 44 Books 46 Letters 51 The Contributors 55 Getting in Touch 56 Mare Nostrum Spyros Harbouris 57 iii Bearded Iris and Lilium candidum iv (M)EDITORIAL Gardening, it is often said, is an activity which we tend to take up later in life. -

Rheology and Conductivity of Phloem
RHEOLOGY AND CONDUCTIVITY OF PHLOEM By SIERRA DAWN BEECHER A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY WASHINGTON STATE UNIVERSITY Molecular Plant Sciences JULY 2016 © Copyright by SIERRA DAWN BEECHER, 2016 All Rights Reserved © Copyright by SIERRA DAWN BEECHER, 2016 All Rights Reserved To the Faculty of Washington State University: The members of the Committee appointed to examine the dissertation of SIERRA DAWN BEECHER find it satisfactory and recommend that it be accepted. ____________________________________ Michael Knoblauch, Ph.D. Chair ____________________________________ Asaph Cousins, Ph.D. ____________________________________ Andrei Smertenko, Ph.D. ____________________________________ Eric Shelden, Ph.D. ii Acknowledgements I have been very fortunate to have had a lot of excellent support with these projects. My advisor, Michael Knoblauch helped me design experiments to address the questions I was most curious about, and generously made available all tools and technology to carry them out. Dan Froelich shared many of the techniques he had developed for in vivo confocal imaging, which were the foundation for the methods that I developed to do cold stimulation experiments. Dan Mullendore graciously shared many insights, and technical tricks about new possibilities presented by the Leica SP8. These were used for fluorescence lifetime imaging to measure the viscosity of translocating phloem sap. Tim Ross-Elliot was always generous with his methods, and helped me develop better molecular biology skills. All members of the Knoblauch Lab, and my committee members, Eric Shelden, Andrei Smertenko, and Asaph Cousins have inspired me with their passion and intelligence. Michael Neff has also been very supportive and helpful. -

Plant Sale 2017 Descriptions Common Name Botanical Name Type Height Light Soil Type Comments Small-Medium, Woody Shrub with Fine Textured, Leafy, Pendulous Branches
Plant Sale 2017 Descriptions Common Name Botanical Name Type Height Light Soil Type Comments Small-medium, woody shrub with fine textured, leafy, pendulous branches. Attractive and colorful hanging flowers with yellow petals surrounded by red calyces all season. Good in hanging Trailing Flowering Maple Abutilon megapotamicum Tropical 18-24 in. Full to part sun Average well drained baskets and combos. Large dramatic species with pink toned new foliage hardening into rich green arching fronds. Grows great in our hot humid summers, but must have high humidity to perform well as a houseplant. Grow in a large Wardian Case as its home (this is far too large for a small or narrow terrarium); nonetheless, if given a bright windowsill and a carefully tended pebble tray, it will also become an impressive specimen given time and humidity as a houseplant. Always a star in any hobby greenhouse. Always a popular and much sought after dramatic rarer plant! Fern, Maidenhair Silver Dollar Adiantum peruvianum Tropical 18-24 in. Part Shade to Shade Moist well drained A stunning Maidenhair that reaches 2-3 feet tall. The bright green leaflets are carried on glossy black stipes making for a dramatic display. Grows much the same as Silver Dollar Maidenhair fern. Fern, Maidenhaier Giant Adiantum polyphyllum Tropical 24-32 in. Part Shade to Shade Moist well drained Plant Sale 2017 Descriptions Common Name Botanical Name Type Height Light Soil Type Comments The delicate fronds and airy appearance of this little fern belie its character, because it is a trooper. Unlike most hardy maidenhair ferns, it's also evergreen. -

Rare Plants: Choice Flower Seeds Cacti and Succulents
Historic, archived document Do not assume content reflects current scientific knowledge, policies, or practices c* DESCRIPTIVE CATALOGUE 1911 Rare Plants Choice Flower Seeds CACTI AND SUCCULENTS Theodosia B. Shepherd Co. Ventura, California u. s. A. Directions for Seed Sowing The first requisite for seed beds and boxes is preparation of the soil, which should be mellow and friable, a mixture of loam, sand and thoroughly rotted manure or leaf mould. Annuals such as Eschscholtzias, Poppies, Mignonette, Centaureas, Candytuft, Calliopsis, Zinnias, Marigolds, etc,, should be planted where they are to grow, and thinned out when two or three inches high, so each plant may have room to develop. Cosmos can be planted the same way and transplanted if too thick. Petunias, Pansies, Stocks, Asters, Carnations, Dianthus, Daisies, Browallia, Dahlias, Geraniums, Heliotrope and many others are best sown in carefully prepared seed beds or cold frames, and transplanted when two or three inches high. Choose the afternoon of a cool day; have the soil moist and mellow^ so that it crumbles fine in the hand. Do not allow the air to dry out the fine roots, cover them with paper or cloth, as a moment or two of exposure may cause loss. See that the soil comes in between the roots,—use the fingers for this, do not pack them down all together. It is most important that the soil should be pressed down firmly after setting out, so as to hold the plants firmly in the ground, and also to keep out sun and air. Water carefully and deep, and when the ground settles, draw earth over the wet ground to prevent soil from packing. -

Flora Mesoamericana, Volume 5 (1), Page 1 of 73
Flora Mesoamericana, Volume 5 (1), page 1 of 73 First published on the Flora Mesoamericana Website, 21 Dec. 2011. Updated 25 Mar. 2014. 222. PLANTAGINACEAE Antirrhinaceae Pers., Aragoaceae D. Don, Callitrichaceae Link, Chelonaceae Martinov, Digitalidaceae Martinov, Ellisiophyllaceae Honda, Erinaceae Duvau, Globulariaceae DC., Gratiolaceae Martinov, Hemimeridaceae Doweld, Hippuridaceae Vest, LinariaceaeBrecht. & J. Presl, Littorellaceae Gray, Oxycladaceae Schnizl., Psylliaceae Horan., Scopariaceae Link, Sibthorpiaceae D. Don, Trapellaceae Honda & Sakis., Veronicaceae Cassel Family description and key by M.J.M. Christenhusz. Usually herbaceous annual or perennials, sometimes shrubs, small trees, submerged aquatics or vines. Leaves alternate and spiral or opposite, simple or compound. Inflorescences variable, racemose top cymose, solitary or compound, terminally or axillary. Flowers usually 4- or 5-merous. Calyx shallowly to deeply 4- or 5-lobed or - parted. Corolla open and lobed or cochleate, often bilabiate and sometimes spurred or saccate, the tube sometimes closed by an inflated palate. Stamens 5-8, usually inserted in the corolla, the thecae parallel, end-to-end, or sagittate. Styles various, simple to branched; stigma flattened, capitate or bilobed. Fruit usually a septicidal, sometimes a loculicidal, poricidal or circumscissile capsule. Seeds usually numerous. c. 90 gen., 1700 spp. Worldwide, mostly temperate. It is not easy distinguishing between Plantaginaceae s.l. and other families in Lamiales. The frequent absence of regular partitions in the heads of glandular hairs and septicidal capsule dehiscence is however unusual among Lamiales, although this is by no means present in all genera of the Plantaginaceae (most notably these characters are not found in the type genus Plantago), and also not unique to it. -

Annual Climbers
ANNUAL CLIMBERS Final Trials Report 2009 Trials Office The Royal Horticultural Society Garden, Wisley, Woking, Surrey, GU23 6QB Annual Climbers from Seed Final Report 2009 - Trial 1842 1 RHS Trial of Annual Climbers Objectives The objectives of the trial were: • To recommend the Award of Garden Merit [AGM] to cultivars considered excellent for ordinary garden use. • To demonstrate the range of Annual Climbers currently available from seed Judging The Floral Trials Subcommittee assessed entries in the trial for the Award of Garden Merit on 21/4, 9/6, 23/6, 7/7, 21/7, 18/8 and 1/9 (2009), using the following criteria: • Length of flowering period • Colour • Floriferousness • Impact • Habit • Uniformity of single colours • Balance of mixtures • Trueness to flower shape and form Entries There were 115 entries in the trial, submitted by various seed companies throughout the United Kingdom and Europe. Cultivation Seed was sown under glass in three batches relating to the ease of germination and speed of growing on. The first batch of seed which included entries of the following genera: Clematis, Dicentra, Eccremocarpus, Humulus, Lathyrus, Melothria, Mutisia, Rhodociton, Thunbergia, Vigna was sown on 13 March 2009. The second batch of seed which included Adlumia, Asarina, Lophospermum, Petunia was sown 27 March. The final batch including Cobaea, Dolichos, Ipomoea, Phaseolus, Tropaeolum was sown on 14 April. Seedlings were planted out in May 2009. Cages 7 feet tall were put in place for the plants to grow up. Pest and Disease Aphid was noted to be present in the trial in June 2009. This was treated by spraying with Phantom (Pirimicarb). -

RHS the Garden Magazine Index 2019
GardenThe INDEX 2019 Volume 144, Parts 1–12 Index 2019 January 2019 February 2019 March 2019 April 2019 May 2019 June 2019 1 2 3 4 5 6 Coloured numbers lasiocarpa Adamson 4: 11, 11 sanpedroensis 1: 8, 8 Allium alpine in bold before the page var. arizonica 12: 54, 55 African plants and Aglaonema ‘Silver ‘Blue Dean’ 9: 79, 79 beds, outdoor at RHS number(s) denote the nordmanniana 12: 52, gardens, by John Queen’ 1: 25, 25 hollandicum ‘Purple Garden Harlow Carr part number (month). 52–53, 53, 54, 55, 56, 56 Grimshaw 3: 119 Ainscough, Daniel, on: Sensation’ 5: 20, 20–21 4: 91, 92 Each part is paginated procera 12: 54, 55 agapanthus gall midge edging for paths and allotment conservation by separately. Acacia baileyana 7: 22, 22 borders 8: 56–57, 56 getting the best from, Scottish Rock ‘Purpurea’ 1: 90 Agapanthus, RHS Plant Akebia longeracemosa by Melissa Mabbitt Garden Club 6: 13 Numbers in italics Acanthus mollis ‘Hollard’s Trial of, by Christine 10: 34 9: 82–84 stack of pots, make a denote an image. Gold’ 11: 69, 70 Skelmersdale 7: 36–41 Albizia julibrissin f. rosea giving up, by Lia 2: 24–25 Acer Agapanthus 2: 45 Leendertz 6: 119 Alpine House at RHS Where a plant has a x conspicuum ‘African Skies’ 7: 38, 39 Alcea holders, diversity of, Garden Harlow Carr Trade Designation ‘Phoenix’ 1: 21, 21 ‘Alan Street’ 7: 38, 39 ficifolia 7: 60–61 by Gary Atkinson 4: 91–92, 91, 92 (also known as a selling japonicum ‘Vitifolium’ ‘Ballerina’ 7: 38, 39 rosea ‘Nigra’ 6: 22 7: 62–66 alpines at RHS Garden name) it is typeset in 5: 87, 88 ‘Blue Ice’ 7: 38, 39 Alchemilla mollis 7: 10, soil, lead in 10: 11 Harlow Carr, by Kaye a different font to negundo ‘Winter ‘Eggesford Sky’ 7: 38, 39 10; 10: 55 Allotment Project, The Collings 4: 91–94 distinguish it from the Lightning’ 1: 38 Ever White (‘WP001’) Alexander-Sinclair, 3: 123 Alstroemeria Inticancha cultivar name (shown palmatum 4: 70, 74; 7: 38, 39 James, on: allotments in Bristol, Bryce (‘Tesbryce’) 9: 19, in ‘Single Quotes’).