Ischnura Elegans, Coenagrionide) in the Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Impacts of Urbanisation on the Ecology and Evolution of Dragonflies and Damselflies (Insecta: Odonata)
The impacts of urbanisation on the ecology and evolution of dragonflies and damselflies (Insecta: Odonata) Giovanna de Jesús Villalobos Jiménez Submitted in accordance with the requirements for the degree of Doctor of Philosophy (Ph.D.) The University of Leeds School of Biology September 2017 The candidate confirms that the work submitted is her own, except where work which has formed part of jointly-authored publications has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. The work in Chapter 1 of the thesis has appeared in publication as follows: Villalobos-Jiménez, G., Dunn, A.M. & Hassall, C., 2016. Dragonflies and damselflies (Odonata) in urban ecosystems: a review. Eur J Entomol, 113(1): 217–232. I was responsible for the collection and analysis of the data with advice from co- authors, and was solely responsible for the literature review, interpretation of the results, and for writing the manuscript. All co-authors provided comments on draft manuscripts. The work in Chapter 2 of the thesis has appeared in publication as follows: Villalobos-Jiménez, G. & Hassall, C., 2017. Effects of the urban heat island on the phenology of Odonata in London, UK. International Journal of Biometeorology, 61(7): 1337–1346. I was responsible for the data analysis, interpretation of results, and for writing and structuring the manuscript. Data was provided by the British Dragonfly Society (BDS). The co-author provided advice on the data analysis, and also provided comments on draft manuscripts. -

Dragonf Lies and Damself Lies of Europe
Dragonf lies and Damself lies of Europe A scientific approach to the identification of European Odonata without capture A simple yet detailed guide suitable both for beginners and more expert readers who wish to improve their knowledge of the order Odonata. This book contains images and photographs of all the European species having a stable population, with chapters about their anatomy, biology, behaviour, distribution range and period of flight, plus basic information about the vagrants with only a few sightings reported. On the whole, 143 reported species and over lies of Europe lies and Damself Dragonf 600 photographs are included. Published by WBA Project Srl CARLO GALLIANI, ROBERTO SCHERINI, ALIDA PIGLIA © 2017 Verona - Italy WBA Books ISSN 1973-7815 ISBN 97888903323-6-4 Supporting Institutions CONTENTS Preface 5 © WBA Project - Verona (Italy) Odonates: an introduction to the order 6 WBA HANDBOOKS 7 Dragonflies and Damselflies of Europe Systematics 7 ISSN 1973-7815 Anatomy of Odonates 9 ISBN 97888903323-6-4 Biology 14 Editorial Board: Ludivina Barrientos-Lozano, Ciudad Victoria (Mexico), Achille Casale, Sassari Mating and oviposition 23 (Italy), Mauro Daccordi, Verona (Italy), Pier Mauro Giachino, Torino (Italy), Laura Guidolin, Oviposition 34 Padova (Italy), Roy Kleukers, Leiden (Holland), Bruno Massa, Palermo (Italy), Giovanni Onore, Quito (Ecuador), Giuseppe Bartolomeo Osella, l’Aquila (Italy), Stewart B. Peck, Ottawa (Cana- Predators and preys 41 da), Fidel Alejandro Roig, Mendoza (Argentina), Jose Maria Salgado Costas, Leon (Spain), Fabio Pathogens and parasites 45 Stoch, Roma (Italy), Mauro Tretiach, Trieste (Italy), Dante Vailati, Brescia (Italy). Dichromism, androchromy and secondary homochromy 47 Editor-in-chief: Pier Mauro Giachino Particular situations in the daily life of a dragonfly 48 Managing Editor: Gianfranco Caoduro Warming up the wings 50 Translation: Alida Piglia Text revision: Michael L. -

Download Download
The Distribution and Relative Seasonal Abundance of the Indiana Species of Agrionidae (Odonata: Zygoptera) B. Elwood Montgomery, Purdue University In a previous paper (Montgomery, 1942) the relative seasonal abundance of the adults of the 16 species of Enallagma known from Indiana, based upon the frequency of collection during 41 years (1900- 1940 inclusive) was indicated. That study is extended here to include the remaining 16 species of the family Agrionidae recorded from the state. <I3jXEEEKE> Fig. 1. The range of the flight season (or period of adult life) and the relative seasonal abundance of 16 species of Agrionidae (genera Argia, Nehalen- nia, Amphiagrion, Chromagrion, Ischnura and Anonialagrion ) in Indiana. Col- lections made from 1900 to 1940 inclusive were tabulated by thirds of months and the graphs constructed from the resulting frequency distributions. Numbers near each bar indicate the number of collections of each species in each third of a month ; where no number is given the number of collections is one. 179 180 Indiana Academy of Science Records of almost all Odonata collected or observed in Indiana since 1900 have been preserved in the note books of the late E. B. Williamson and of the author. The records for the species of the genera Argia, Nehalennia, Amphiagrion, Chromagrion, Ischnura, and Anomalagrion have been tabulated and the accompanying chart (Figure 1) indicates the relative abundance of the different species throughout the season of adult flight. As this study applies only to adults, all references to seasonal range and abundance in this paper refer to the period of adult flight. The records of captures (or observations) were tabulated by thirds of months and the time-frequency graph for each species was con- structed by plotting the frequency for each third at the midpoint (5th, 15th and 25th of the month, respectively) of the third of the time axis. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Argia the News Journal of the Dragonfly Society of the Americas
ISSN 1061-8503 TheA News Journalrgia of the Dragonfly Society of the Americas Volume 22 17 December 2010 Number 4 Published by the Dragonfly Society of the Americas http://www.DragonflySocietyAmericas.org/ ARGIA Vol. 22, No. 4, 17 December 2010 In This Issue .................................................................................................................................................................1 Calendar of Events ......................................................................................................................................................1 Minutes of the 2010 Annual Meeting of the Dragonfly Society of the Americas, by Steve Valley ............................2 2010 Treasurer’s Report, by Jerrell J. Daigle ................................................................................................................2 Enallagma novaehispaniae Calvert (Neotropical Bluet), Another New Species for Arizona, by Rich Bailowitz ......3 Photos Needed ............................................................................................................................................................3 Lestes australis (Southern Spreadwing), New for Arizona, by Rich Bailowitz ...........................................................4 Ischnura barberi (Desert Forktail) Found in Oregon, by Jim Johnson ........................................................................4 Recent Discoveries in Montana, by Nathan S. Kohler ...............................................................................................5 -
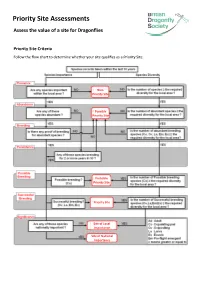
Priority Site Assessments Assess the Value of a Site for Dragonflies
Priority Site Assessments Assess the value of a site for Dragonflies Priority Site Criteria Follow the flow chart to determine whether your site qualifies as a Priority Site. Non- Priority Site Possible Priority Site Probable Priority Site Priority Site Site of Local Importance Site of National Importance Nationally Important Species List Azure Hawker, Aeshna caerulea Scarce Chaser, Libellula fulva Brilliant Emerald, Somatochlora metallica Scarce Emerald Damselfly, Lestes dryas Common Clubtail, Gomphus vulgatissimus Southern Damselfly, Coenagrion mercuriale Northern Emerald, Somatochlora arctica Small Red Damselfly, Ceriagrion tenellum Norfolk Hawker, Aeshna isosceles Variable Damselfly, Coenagrion pulchellum Northern Damselfly, Coenagrion hastulatum White-faced Darter, Leucorrhinia dubia Scarce Blue-tailed Damselfly, Ischnura pumilio Locally Important Species List and Diversity Criteria The diversity criteria is the minimum number of species that need to have been recorded within the site, within the last 10 years, for it to qualify. Counties Locally Important Species Diversity criteria Cornwall White-legged Damselfly, Platycnemis pennipes 11 Isles of Scilly Ruddy Darter, Sympetrum sanguineum Devon White-legged Damselfly, Platycnemis pennipes 14 Red-eyed Damselfly, Erythromma najas Hairy Dragonfly, Brachytron pratense Downy Emerald, Cordulia aenea Ruddy Darter, Sympetrum sanguineum Dorset White-legged Damselfly, Platycnemis pennipes 14 Red-eyed Damselfly, Erythromma najas Downy Emerald, Cordulia aenea Somerset Common Hawker, Aeshna juncea -

Ohio Damselfly Species Checklist
Ohio Damselfly Species Checklist Ohio has ~51 species of damselflies (Zygoptera). This is a statewide species checklist to encourage observations of damselflies for the Ohio Dragonfly Survey. Please submit photo observations to iNaturalist.org. More information can be found on our survey website at u.osu.edu/ohioodonatasurvey/ Broad Winged Damselflies (Calopterygidae) 1 Appalachian Jewelwing Calopteryx angustipennis 2 River Jewelwing Calopteryx aequabilis State Endangered 3 Ebony Jewelwing Calopteryx maculata 4 American Rubyspot Hetaerina americana 5 Smoky Rubyspot Hetaerina titia Pond Damselflies (Coenagrionidae) 6 Eastern Red Damsel Amphiagrion saucium 7 Blue-fronted Dancer Argia apicalis 8 Seepage Dancer Argia bipunctulata State Endangered 9 Powdered Dancer Argia moesta 10 Blue-ringed Dancer Argia sedula 11 Blue-tipped Dancer Argia tibialis 12 Dusky Dancer Argia translata 13 Violet Dancer Argia fumipennis violacea 14 Aurora Damsel Chromagrion conditum 15 Taiga Bluet Coenagrion resolutum 16 Turquoise Bluet Enallagma divagans 17 Hagen's Bluet Enallagma hageni 18 Boreal Bluet Enallagma boreale State Threatened 19 Northern Bluet Enallagma annexum State Threatened 20 Skimming Bluet Enallagma geminatum 21 Orange Bluet Enallagma signatum 22 Vesper Bluet Enallagma vesperum 23 Marsh Bluet Enallagma ebrium State Threatened 24 Stream Bluet Enallagma exsulans 25 Rainbow Bluet Enallagma antennatum 26 Tule Bluet Enallagma carunculatum 27 Atlantic Bluet Enallagma doubledayi 1 28 Familiar Bluet Enallagma civile 29 Double-striped Bluet Enallagma basidens -

Zygoptera: Coenagrionidae) Has an Andromorphic Female, and Another Suggestion to Modify the Terminology of Female Color Polymorphism in Odonata
International Journal of Odonatology 2 (1): 101-103, 1999. © 1999 Backhuys Publishers. 101 ISCHNURA PERPARVA MCLACHLAN (ZYGOPTERA: COENAGRIONIDAE) HAS AN ANDROMORPHIC FEMALE, AND ANOTHER SUGGESTION TO MODIFY THE TERMINOLOGY OF FEMALE COLOR POLYMORPHISM IN ODONATA DENNIS R. PAULSON Slater Museum of Natural History, University of Puget Sound, Tacoma, WA 98416 U.S.A.- Email: [email protected] This paper is dedicated to Philip S. Corbet on the occasion of his 70th birthday. Received 30 June 1998; revised 22 Februari 1999; accepted 22 March 1999 Key words: coloration, damselfly, /schnura perparva, Odonata, polymorphism, Zygoptera Polymorphism in lschnura perparva There is considerable theoretical interest in the polymorphism shown by females of some species ofOdonata (Johnson, 1975 Robertson, 1985 Hinnekint, 1987 Cordero, 1992; Fincke 1994). This polymorphism is especially conspicuous in many species of lschnura, in which it is correlated with other traits (Robinson & Allgeyer 1996). Robinson & Allgeyer (1996) and Westfall & May (1996) listed all 12 North American /schnura species as polymorphic, with the exception of /. demorsa, /. hastata, I. perparva, and /. posita, which were considered monomorphic. However, I recently examined all 235 female /. perparva in my collection and found two andromorphic individuals, along with 16 immature (orange) gynomorphic, 216 mature (pruinose ), and one clearly intermediate between the last two. The andromorphic females were collected in Washington state, from a pond 0.5 mi E Beverly, Grant Co., 5 August 1972, and a lake west of Lower Hampton Lake, Grant Co., 27 July 1973. The andromorphic females, both quite young, differ from my sample of gynomorphic immatures and the detailed description of the female by Kennedy (1915) in three ways, involving both color and pattern. -

Dragonflies and Damselflies of Peninsular India-A Field Guide. E-Book of Project Lifescape
K.A.Subramanian (2005) Dragonflies and Damselflies of Peninsular India-A Field Guide. E-Book of Project Lifescape. Centre for Ecological Sciences, Indian Institue of Science and Indian Academy of Sciences, Bangalore, India. 118 pages. Copyright K.A.Subramanian, 2005. 75 K.A.Subramanian (2005) Dragonflies and Damselflies of Peninsular India-A Field Guide. E-Book of Project Lifescape. Centre for Ecological Sciences, Indian Institue of Science and Indian AcademyMARSH of Sciences, Bangalore, DAR India. 118TS pages. Copyright (FAMIL K.A.Subramanian,Y 2005.: COENAGRIONIDAE) MARSH DARTS (FAMILY: COENAGRIONIDAE) Marsh darts are slender and small damselflies with varied colouration. These non-iridescent damselflies rest with wings closed over their body. The wings are transparent and rounded at the tip. The long and slender abdomen is slightly longer than the hind wing. Some of the smallest damselflies like the Golden Dartlet (Ischnura aurora) is from this family. Marsh Darts are found throughout the world. World over, this family is represented by about 1147 species. Within Indian limits, 65 species are known and in peninsular India 25 species are recorded. The marsh darts breed in a variety of aquatic habitats like ponds, marshes, streams and Photo:E.Kunhikrishnan rivers. Though most of the species are closely associated with aquatic habitats, some Golden Dartlets mating species like the Common Marsh Dart (Ceriagrion coromandelianum) can be found far away from any aquatic habitat. Photo:K.A.Subramanian Golden Dartlet- male 76 K.A.Subramanian (2005) Dragonflies and Damselflies of Peninsular India-A Field Guide. E-Book of Project Lifescape. Centre for Ecological Sciences, Indian Institue of Science and Indian Academy of Sciences, Bangalore, India. -

Dragonflies and Damselflies Havasu National Wildlife Refuge
U.S. Fish & Wildlife Service Dragonflies and Damselflies Havasu National Wildlife Refuge Dragonfly and damselfly at Havasu National Wildlife Refuge There are twenty-five dragonfly and damselfly species listed at the 37,515 acre Havasu National Wildlife Refuge, one of more than 540 refuges throughout the United States. These National Wildlife Refuges are administered by the Department of the Interior, Fish and Wildlife Service. The Fish and Wildlife Service mission is to work with others “to conserve fish and wildlife and their habitat.” General Information Havasu National Wildlife Refuge encompasses 37,515 acres adjacent to the Colorado River. Topock Marsh, Topock Gorge, and the Havasu Wilderness constitute the three major portions of the refuge. Dragonflies, an important Male blue-ringed dancer sedula indicator of water quality, can be found © Dave Welling Photography on the refuge, primarily in Topock Marsh Libellula luctuosa Tramea onusta and Topock Gorge. Dragonflies can be Widow Skimmer Red Saddlebags viewed on the refuge year-round, with hot, sunny days providing some of the L. pulchella Pond Damsels–Dancers (Coenagrionidae) best viewing. Sixty-three dragonfly and Twelve-spotted Skimmer Argia moesta damsel species have been identified in Powdered Dancer Mohave County, Arizona. Visitors are L. saturate encouraged to contact refuge staff with a Flame Skimmer Argia sedula description or photograph, if an unlisted Blue-ringed Dancer species is observed. Pachydiplax longipennis Blue Dasher Enallagma civile Family Familiar Bluet Scientific -

The Dragonfly Delusion: Why It Is Essential to Sample Exuviae To
J Insect Conserv DOI 10.1007/s10841-010-9281-7 ORIGINAL PAPER The dragonfly delusion: why it is essential to sample exuviae to avoid biased surveys Eva M. Raebel • Thomas Merckx • Philip Riordan • David W. Macdonald • David J. Thompson Received: 28 October 2009 / Accepted: 12 February 2010 Ó Springer Science+Business Media B.V. 2010 Abstract Odonate populations and species numbers are with non-territorial mate-location strategies. Odonate bio- declining globally. Successful conservation requires sound diversity monitoring would benefit from applying the best assessments of both odonate distributions and habitat survey method (exuviae) to avoid wasting valuable financial requirements. Odonates have aquatic (larval) and terrestrial resources while providing unbiased data, necessary to (adult) stages, but most surveys that are used to inform achieve conservation objectives. conservation managers are undertaken of the adult stage. This study investigates whether this bias towards adult Keywords Odonata Á Survey methods Á Exuviae Á records in odonate recording is misinterpreting the envi- Ecological traps Á Conservation ronmental quality of sites. The habitat focus is farmland ponds, a key feature of agricultural landscapes. We tested whether or not, adult, larval and exuvial surveys lead to Introduction similar conclusions on species richness and hence on pond quality. Results showed that pond surveys based upon larvae Odonates are declining worldwide due to the loss and and exuviae are equally suitable for the reliable assessment deterioration of their habitat (Clausnitzer et al. 2009), and of presence/absence of odonates, but that adult surveys are nearly 15% of known species are currently threatened with not interchangeable with surveys of larvae/exuviae. -

Including a Third Species of Ischnura Damselfly
Errata and Addenda: Updated Illustrated Checklist of Dragonflies of the UAE – including a third species of Ischnura damselfly by Robert W. Reimer, Gary R. Feulner and Richard J. Hornby Our paper titled “An Updated Illustrated Checklist of Errata Dragonflies of the UAE” (Feulner et al. 2007), published in Tribulus Vol. 17, stimulated valuable comment and Among the photographs which illustrated the inquiry as well as further investigations by the authors checklist, we made the decision to include images of a themselves. These confirmed errors in the identifications number of atypical forms seen over the years, which attributed to several photographs, which are corrected were not always readily identifiable. Subsequent below. comment by international experts has confirmed the In addition, continuing research and field studies by value of that decision from a heuristic standpoint, but also Reimer have revealed the presence of a third species of emphasises that we should have been more circumspect Ischnura damselfly, as well as the possible presence of in assigning even tentative identifications. a further dragonfly species observed in the coastal Three of our photographs, all of immature individuals, environment in neighbouring Oman. These have now been more authoritatively identified and a note developments are reported below, along with certain is made here of the corrections. K-D.B. Dijkstra, additional information likely to be of interest to those Wolfgang Schneider and Laurent Juillerat have all written studying the Odonata of the UAE and northern Oman, to indicate their views as follows (all Figures referenced including tips for field discrimination between Ischnura are reproduced here): damselfly species.