Evolutionary History and Speciation of the Genus Tragopan
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CITES Cop16 Prop. 15 IUCN-TRAFFIC Analysis (PDF
Ref. CoP16 Prop. 15 Deletion of Grey Junglefowl Gallus sonneratii from Appendix II Proponent: Switzerland, as Depositary Government, at the Request of the Animals Committee (prepared by New Zealand) Summary: The Grey Junglefowl Gallus sonneratii is endemic to India and inhabits subtropical and tropical moist forests, bamboo thickets, open woodlands and dry deciduous shrubland. The species has a wide range, estimated at around 1 million km2. It is believed to be affected by habitat loss and by some illegal hunting for its meat for domestic consumption. Good populations are likely now to be mainly confined to protected areas. The overall population is believed likely to be declining, though not at a rate fast enough to merit classifying the species as threatened. It was assessed as of Least Concern in 2012 by BirdLife International. Gallus sonneratii was one of several species of Galliform included in Appendix II in 1975 owing to concerns about the international trade in their feathers – the males possess long neck hackles (elongated feathers) with very distinctive patterning, which are in demand for making fishing flies. In the period 2000–2010, nearly 240 000 G. sonneratii feathers were recorded in the CITES trade database as in international trade; 99% of these were reported as coming from captive- bred birds and virtually all exported from non-range States. Over half were exported from the UK to the USA in 2001. Very little trade in feathers has been reported since 2004. There is a small amount of trade in live, captive-bred birds. The species is reported to be easy to keep in captivity. -

Status of Galliformes in Pipar Pheasant Reserve and Santel, Annapurna, Nepal
Status of Galliformes in Pipar Pheasant Reserve and Santel, Annapurna, Nepal 1 2 3 4 LAXMAN P. POUDYAL *, NAVEEN K. MAHATO , PARAS B. SINGH , POORNESWAR SUBEDI , HEM S. BARAL 5 and PHILIP J. K. MCGOWAN 6 1 Department of National Parks and Wildlife Conservation, PO Box 860, Babarmahal, Kathmandu, Nepal. 2 Red Panda Network – Nepal, PO Box 21477, Kathmandu, Nepal. 3 Biodiversity Conservation Society, PO Box 24304, Kathmandu, Nepal. 4 Department of Forests, Kathmandu, Nepal. 5 Bird Conservation Nepal, PO Box 12465, Lazimpat, Kathmandu, Nepal. 6 World Pheasant Association, Newcastle University Biology Field Station, Close House Estate, Heddon on the Wall, Newcastle upon Tyne, NE15 0HT UK. *Correspondence author - [email protected] Paper presented at the 4 th International Galliformes Symposium, 2007, Chengdu, China. Abstract The Galliformes of Pipar have been surveyed seven times between 1979 and 1998. The nearby area of Santel was surveyed using comparable methods in 2001. In continuance of the long- term monitoring at Pipar and to provide a second count at Santel, dawn call counts were conducted in both areas, using the same survey points as previous surveys, between 29 th April and 9 th May 2005. The aim of those surveys was to collect information on the status of pheasants and partridges and to look for any changes over time. In both areas, galliform numbers were higher in 2005 than in previous surveys, for most species. For each species there was no evidence of decline since the first counts were conducted nearly 30 years ago. Both areas provide good habitat for Galliformes and disturbance is not a serious issue. -

Status of the Vulnerable Western Tragopan (Tragopan Melanocephalus) in Pir-Chinasi/Pir- Hasimar Zone, Azad Jammu & Kashmir, Pakistan
Status of Western Tragopan in Pir-Chinasi/Pir-Hasimar Zone of Jhelum Valley Status of the Vulnerable Western Tragopan (Tragopan melanocephalus) in Pir-Chinasi/Pir- Hasimar zone, Azad Jammu & Kashmir, Pakistan. Final Report (2011-12) Muhammad Naeem Awan* Project sponsor: Himalayan Nature Conservation Foundation Oriental Bird Club, UK Status of Western Tragopan in Pir-Chinasi/Pir-Hasimar Zone of Jhelum Valley Suggested Citation: Awan, M. N., 2012. Status of the Vulnerable Western Tragopan (Tragopan melanocephalus) in Jhelum Valley (Pir-Chinasi/Pir-Hasimar zone), Azad Jammu & Kashmir, Pakistan. Final Progress Report submitted to Oriental Bird Club, UK. Pp. 18. Cover Photos: A view of survey plot (WT10) in Pir-Chinasi area, Muzaffarabad, Azad Kashmir, Pakistan, where Tragopan was confirmed. Contact Information: Muhammad Naeem Awan Himalayan Nature Conservation Foundation (HNCF) Challa Bandi, Muzaffrarabad Azad Jammu & Kashmir Pakistan. 13100 [email protected] Status of Western Tragopan in Pir-Chinasi/Pir-Hasimar Zone of Jhelum Valley Abbreviations and Acronyms AJ&K : Azad Jammu & Kashmir HNCF: Himalayan Nature Conservation Foundation PAs: Protected Areas PCPH: Pir-Chinasi/Pir-Hasimar A A newly shot Tragopan B View of PCPH C Monal Pheasant’s head used as decoration in one home in the study area D Summer houses in the PCPH Status of Western Tragopan in Pir-Chinasi/Pir-Hasimar Zone of Jhelum Valley EXECUTIVE SUMMARY Study area, Pir-Chinasi/Pir-Hasimar (PCPH) zone (34.220-460N, 73.480-720E) is a part of the Western Himalayan landscape in Azad Kashmir, Pakistan; situated on both sides along a mountain ridge in the northeast of Muzaffarabad (capital town of AJ&K). -

Hybridization & Zoogeographic Patterns in Pheasants
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Paul Johnsgard Collection Papers in the Biological Sciences 1983 Hybridization & Zoogeographic Patterns in Pheasants Paul A. Johnsgard University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/johnsgard Part of the Ornithology Commons Johnsgard, Paul A., "Hybridization & Zoogeographic Patterns in Pheasants" (1983). Paul Johnsgard Collection. 17. https://digitalcommons.unl.edu/johnsgard/17 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Paul Johnsgard Collection by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. HYBRIDIZATION & ZOOGEOGRAPHIC PATTERNS IN PHEASANTS PAUL A. JOHNSGARD The purpose of this paper is to infonn members of the W.P.A. of an unusual scientific use of the extent and significance of hybridization among pheasants (tribe Phasianini in the proposed classification of Johnsgard~ 1973). This has occasionally occurred naturally, as for example between such locally sympatric species pairs as the kalij (Lophura leucol11elana) and the silver pheasant (L. nycthelnera), but usually occurs "'accidentally" in captive birds, especially in the absence of conspecific mates. Rarely has it been specifically planned for scientific purposes, such as for obtaining genetic, morphological, or biochemical information on hybrid haemoglobins (Brush. 1967), trans ferins (Crozier, 1967), or immunoelectrophoretic comparisons of blood sera (Sato, Ishi and HiraI, 1967). The literature has been summarized by Gray (1958), Delacour (1977), and Rutgers and Norris (1970). Some of these alleged hybrids, especially those not involving other Galliformes, were inadequately doculnented, and in a few cases such as a supposed hybrid between domestic fowl (Gallus gal/us) and the lyrebird (Menura novaehollandiae) can be discounted. -

Why Do Eared-Pheasants of the Eastern Qinghai-Tibet Plateau Show So Much Morphological Variation?
Bird Conservation International (2000) 10:305–309. BirdLife International 2000 Why do eared-pheasants of the eastern Qinghai-Tibet plateau show so much morphological variation? XIN LU and GUANG-MEI ZHENG Summary It is known that White Eared-pheasants Crossoptilon crossoptilon drouyni interbreed widely with Tibetan Eared-pheasants C. harmani at the boundary of their ranges. A new hybrid zone has been found recently in eastern Tibet, far away from the boundary of the parental species’ ranges. Based on ecological observations of eared-pheasants and the geographical history and pattern of modern glaciers, we have attributed the complex morphological variation of eared-pheasants and the high biodiversity of the eastern Qinghai-Tibet plateau to its varied geography. Introduction The eared-pheasant genus Crossoptilon is endemic to China and includes four species. The Brown Eared-pheasant C. mantchuricum is found in northern China and the Blue Eared-pheasant C. auritum occurs on the plateau of northern Qinghai-Tibet. These species show no morphological variation and have no described subspecies. The White Eared-pheasant C. crossoptilon shows greater variations in plumage colour and four subspecies have been recognised: crossopti- lon in western Sichuan and south-eastern Tibet, lichiangense in north-western Yunnan, drouyni in the area between the Nujiang River and the Jinsha River, and dolani in Yushu of southern Qinghai. The Tibetan Eared-pheasant C. harmani is restricted to Tibet, north of the main axis of the Himalayas (Ludlow and Kinnear 1944). It was formerly treated as a subspecies of the White Eared-pheasant (Delacour 1977, Cheng et al. 1978), but more recently has been considered a full species (Sibley and Monroe 1990, Cheng 1994), mainly because of its dark blue- grey plumage, which is distinct from the predominately white plumage of the other subspecies. -

MONITORING PHEASANTS (Phasianidae) in the WESTERN HIMALAYAS to MEASURE the IMPACT of HYDRO-ELECTRIC PROJECTS
THE RING 33, 1-2 (2011) DOI 10.2478/v10050-011-0003-7 MONITORING PHEASANTS (Phasianidae) IN THE WESTERN HIMALAYAS TO MEASURE THE IMPACT OF HYDRO-ELECTRIC PROJECTS Virat Jolli, Maharaj K. Pandit ABSTRACT Jolli V., Pandit M.K. 2011. Monitoring pheasants (Phasianidae) in the Western Himalayas to measure the impact of hydro-electric projects. Ring 33, 1-2: 37-46. In this study, we monitored pheasants abundance to measure the impact of a hydro- electric development project. The pheasants abundance was monitored using “call count” and line transect methods during breeding seasons in 2009-2011. Three call count stations and 3 transects were laid with varying levels of anthropogenic disturbance. To understand how the hydro power project could effect the pheasant population in the Jiwa Valley, we monitored it under two conditions; in the presence of hydro-electric project (HEP) con- struction and when human activity significantly declined. The Koklass Pheasant (Pucrasia macrolopha), Cheer Pheasant (Catreus wallichi) and Western Tragopan (Tragopan melano- cephalus) were not recorded in Manjhan Adit in 2009. During 2010 and 2011 springs, the construction activity was temporarily discontinued in Manjhan Adit. The pheasants re- sponded positively to this and their abundance increased near disturbed sites (Manjhan Adit). The strong response of pheasants to anthropogenic disturbance has ecological appli- cation and thus can be used by wildlife management in the habitat quality monitoring in the Himalayan Mountains. V. Jolli (corresponding author), M.K. Pandit, Centre for Inter-disciplinary Studies of Mountain and Hill Environment, Academic Research Building, Patel Road, Univ. of Delhi, Delhi, India, E-mail: [email protected] Key words: call count, anthropogenic disturbance, pheasant, monitoring, hydro-electric project INTRODUCTION Birds have been used extensively in environment and habitat quality monitoring. -
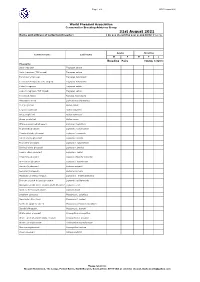
31St August 2021 Name and Address of Collection/Breeder: Do You Closed Ring Your Young Birds? Yes / No
Page 1 of 3 WPA Census 2021 World Pheasant Association Conservation Breeding Advisory Group 31st August 2021 Name and address of collection/breeder: Do you closed ring your young birds? Yes / No Adults Juveniles Common name Latin name M F M F ? Breeding Pairs YOUNG 12 MTH+ Pheasants Satyr tragopan Tragopan satyra Satyr tragopan (TRS ringed) Tragopan satyra Temminck's tragopan Tragopan temminckii Temminck's tragopan (TRT ringed) Tragopan temminckii Cabot's tragopan Tragopan caboti Cabot's tragopan (TRT ringed) Tragopan caboti Koklass pheasant Pucrasia macrolopha Himalayan monal Lophophorus impeyanus Red junglefowl Gallus gallus Ceylon junglefowl Gallus lafayettei Grey junglefowl Gallus sonneratii Green junglefowl Gallus varius White-crested kalij pheasant Lophura l. hamiltoni Nepal Kalij pheasant Lophura l. leucomelana Crawfurd's kalij pheasant Lophura l. crawfurdi Lineated kalij pheasant Lophura l. lineata True silver pheasant Lophura n. nycthemera Berlioz’s silver pheasant Lophura n. berliozi Lewis’s silver pheasant Lophura n. lewisi Edwards's pheasant Lophura edwardsi edwardsi Vietnamese pheasant Lophura e. hatinhensis Swinhoe's pheasant Lophura swinhoii Salvadori's pheasant Lophura inornata Malaysian crestless fireback Lophura e. erythrophthalma Bornean crested fireback pheasant Lophura i. ignita/nobilis Malaysian crestless fireback/Vieillot's Pheasant Lophura i. rufa Siamese fireback pheasant Lophura diardi Southern Cavcasus Phasianus C. colchicus Manchurian Ring Neck Phasianus C. pallasi Northern Japanese Green Phasianus versicolor -

Survey of Western Tragopan, Koklass Pheasant, and Himalayan Monal Populations in the John Corder, World Pheasant Association
60 Indian BIRDS Vol. 6 No. 3 (Publ. 7th August 2010) Survey of Western Tragopan, Koklass Pheasant, and Himalayan Monal populations in the Pheasant Association. World John Corder, Great Himalayan National Park, Himachal Pradesh, India Fig. 1. Himalayan Monal Lophophorus Jennifer R. B. Miller impejanus. Miller, J. R. B. 2010. Survey of Western Tragopan, Koklass Pheasant, and Himalayan Monal populations in the Great Himalayan National Park, Himachal Pradesh, India. Indian BIRDS 6 (3): XXX–XXX. Jennifer R. B. Miller1, Yale School of Forestry & Environmental Studies, 195 Prospect Street, New Haven Connecticut 06511, USA. Email: [email protected] Surveys conducted in the late 1990’s indicated that pheasant populations in the Great Himalayan National Park, Himachal Pradesh, India were declining. In 1999, the government legally notified the park and authorities began enforcing the Indian Wildlife (Protection) Act, banning biomass extraction within park boundaries and reducing human disturbance. Populations of three pheasant species (Western Tragopan, Koklass Pheasant and Himalayan Monal) were subsequently surveyed in the park during the breeding season (April–May) in 2008. Call counts and line transects were used to assess current abundances and gather more information on the characteristics of these species in the wild. Relative abundances of all three species were significantly higher than in previous surveys. Tragopan males began their breeding calls in late April and continued through May whereas Koklass males called consistently throughout the study period. The daily peak calling periods of the two species overlapped, but Tragopan males began calling earlier in the morning than Koklass males. Monals were most often sighted alone or in pairs and larger groups tended to have equal sex composition or a slightly higher number of females than males. -

Western Tragopan, Western Horned Tragopan, Black- Headed Tragopan Local Names: Jujurana (Himachal Pradesh)
Tragopan melanocephalus Common name: Western Tragopan, Western Horned Tragopan, Black- headed Tragopan Local names: Jujurana (Himachal Pradesh) Classification: Kingdom: Animalia Phylum: Chordata Class: Aves Order: Galliformes Family: Phasianidae Genus: Tragopan Species: melavocphalus Profile: The western tragopan is a medium sized brightly coloured pheasant. The males have greyish black feathers with black-bordered white spots and a conspicuous red collar and whattle (fleshy wrinkled fold of skin hanging from the throat). The lappet (flap-like structure at the neck) is bright blue with purple markings while the head is black and has two colourful fleshy “horns”. Females are mainly brownish-grey with none of the colourful appendages seen in males. The upperparts are spotted with black patches with white central streaks. The male body is 70-74 cm long and weighs 1800-2200 g while the female body is 60-62 cm long and weighs 1300-1400 g. The beak is rounded and short with feathers almost touching the nostrils. Lifespan: Data unavailable Distribution: The species is distributed along the Himalayas from Hazara in northern Pakistan to Uttarakhand in India, generally preferring dense montane vegetation. Populations of the western tragopan can be found in Himachal Pradesh and Kashmir in India and in the north West Frontier Province in Pakistan. They mainly inhabit north facing areas with temperate forests (coniferous and deciduous) at an altitudinal range of 2400-2600 m during the breeding season in summer and move to grassy mid-altitudinal regions (1700- 2500 m) in the winter. The preferred habitat includes understorey of temperate, subalpine and broad-leaved forest. The species is endemic to the western Himalayas ranging from Kohistan in north Pakistan up to Himachal Pradesh in northwest India. -

(Tragopan Melanocephalus) in Azad Jammu & Kashmir, Pakistan
Final Report 2015 Conservation action planning for Western Tragopan (Tragopan melanocephalus) in Azad Jammu & Kashmir, Pakistan. By Muhammad Naeem Awan OBC. Grant # P1055 Suggested Citation: Awan, M. N., 2015. Conservation action planning for Western Tragopan (Tragopan melanocephalus) in Azad Jammu & Kashmir, Pakistan Final Progress Report submitted to Oriental Bird Club, UK. Pp.12 PROJECT SUMMARY Western Tragopan (Tragopan melanocephalus) is currently listed as Vulnerable (BirdLife International, 2013) and Azad Jammu & Kashmir (Pakistan) is believed to still hold more important population pockets in other parts of the state. Current project was continuation of efforts to establish the status and distribution of Western Tragopan in AJ&K using the call count technique along with assessment of different threats. Surveys were conducted on the eight newly established plots in the upper part of Neelum valley which has never been surveyed before. In total 2 calling males were recorded from the two survey plots i.e. 42 and 43.1 each. Unexpected heavy rains created hurdles during the surveys which may be resulted into low detection of calls. All the surveys sites are facing heavy anthropogenic pressures and forest cutting either for commercial or local use is at the top. Acknowledgment I am really thankful to Mr. James Goodhart and the Oriental Birds Club for granting me the donation of GBP1500 to continue the surveys and exploring the further sites holding Tragopan population. My special thanks are due to Mr. Francis Buner for his continued guidance throughout the project period and to further help analyze the data to understand the current status of the species in Azad Kashmir. -

China: Sichuan Tour
CHINA: SICHUAN TOUR 21st MAY - 7th JUNE 2019 Temminck’s Tragopan is one of our targets on this trip. www.birdingecotours.com [email protected] 2 | ITINERARY China: Sichuan May-June 2019 Sichuan offers an excellent introduction to birdwatching in China with large numbers of endemic and specialty species, set in some seriously stunning mountain landscapes. The huge, mountainous province of Sichuan is blessed with a range of interesting habitats, and we will ensure that we visit a representative sample of as many of these as possible in our quest to find the region's exciting birds. The tour will start and end in Chengdu, the capital of the province, and we will visit the renowned Longcanggou Forest Park, Labahe Nature Reserve, Wolong National Nature Reserve with Balang Shan, the slopes of Mengbi Shan, and the Baxi Forest areas in our search for birds. We will find many gorgeous game birds, parrotbills, laughingthrushes, and rosefinches during the tour, with particular highlights likely to include Chinese Monal, Blood, White Eared, Blue Eared, Golden, and Lady Amherst’s Pheasants, Tibetan Snowcock, Temminck’s Tragopan, Snow Partridge, Black-necked Crane, Giant Laughingthrush, Firethroat, Golden Bush Robin, Chinese Rubythroat, Siberian Rubythroat, Emei Shan Liocichla, Grandala, Przevalski’s Nuthatch, Pere David’s Tit, Wallcreeper, and Crested Tit-warbler, along with a near endless list of interesting thrushes, robins, and warblers. Throw in some excellent food, friendly local people, and a great infrastructure, and a fantastic, bird-filled trip is on the cards This tour can be combined with our China: 9-day Yunnan pre-tour 2019 tour and/or with our China: 15-day Qinghai extension 2019 tour into a mind-boggling 43-day China mega tour. -

Dramatic Decline of the Vulnerable Reeves's Pheasant Syrmaticus Reevesii, Endemic to Central China
Dramatic decline of the Vulnerable Reeves’s pheasant Syrmaticus reevesii, endemic to central China C HUNFA Z HOU,JILIANG X U and Z HENGWANG Z HANG Abstract The current status and distribution of the regions (Zheng & Wang, 1998; Collar et al., 2001). Reeves’s Vulnerable Reeves’s pheasant Syrmaticus reevesii, endemic pheasant is sensitive to expansion of development and there to central China, is poorly known. To obtain updated is no space for the species to shift its range in central China, information on its status we selected 89 candidate sites in six especially with the high degree of habitat fragmentation in provinces and one municipality in central China and this area (Zheng & Wang, 1998). Previous surveys (Xu et al., conducted interviews and field surveys from April 2011 to 1996; Ma et al., 2009) have indicated declines in the April 2012. Interviews demonstrated the pheasant has distribution and population density of Reeves’s pheasant disappeared from 46% of the surveyed sites. Our results but these surveys did not cover the entire range of the also revealed a population decline at 46 sites, including species or lacked accurate data. protected areas, although population densities in protected In the study reported here we conducted a large-scale areas were higher than those in non-protected areas. Eighty- and intensive survey of Reeves’s pheasant from April 2011 three, 26 and 20% of the surveyed sites had evidence of to April 2012. We aimed to: (1) ascertain the current poaching, habitat loss and use of poison, respectively, which distribution and population status of Reeves’s pheasant in were the three major threats to this species.