Organic Carbon Sources and Sinks in San Francisco Bay: Variability Induced by River Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
0 5 10 15 20 Miles Μ and Statewide Resources Office
Woodland RD Name RD Number Atlas Tract 2126 5 !"#$ Bacon Island 2028 !"#$80 Bethel Island BIMID Bishop Tract 2042 16 ·|}þ Bixler Tract 2121 Lovdal Boggs Tract 0404 ·|}þ113 District Sacramento River at I Street Bridge Bouldin Island 0756 80 Gaging Station )*+,- Brack Tract 2033 Bradford Island 2059 ·|}þ160 Brannan-Andrus BALMD Lovdal 50 Byron Tract 0800 Sacramento Weir District ¤£ r Cache Haas Area 2098 Y o l o ive Canal Ranch 2086 R Mather Can-Can/Greenhead 2139 Sacramento ican mer Air Force Chadbourne 2034 A Base Coney Island 2117 Port of Dead Horse Island 2111 Sacramento ¤£50 Davis !"#$80 Denverton Slough 2134 West Sacramento Drexler Tract Drexler Dutch Slough 2137 West Egbert Tract 0536 Winters Sacramento Ehrheardt Club 0813 Putah Creek ·|}þ160 ·|}þ16 Empire Tract 2029 ·|}þ84 Fabian Tract 0773 Sacramento Fay Island 2113 ·|}þ128 South Fork Putah Creek Executive Airport Frost Lake 2129 haven s Lake Green d n Glanville 1002 a l r Florin e h Glide District 0765 t S a c r a m e n t o e N Glide EBMUD Grand Island 0003 District Pocket Freeport Grizzly West 2136 Lake Intake Hastings Tract 2060 l Holland Tract 2025 Berryessa e n Holt Station 2116 n Freeport 505 h Honker Bay 2130 %&'( a g strict Elk Grove u Lisbon Di Hotchkiss Tract 0799 h lo S C Jersey Island 0830 Babe l Dixon p s i Kasson District 2085 s h a King Island 2044 S p Libby Mcneil 0369 y r !"#$5 ·|}þ99 B e !"#$80 t Liberty Island 2093 o l a Lisbon District 0307 o Clarksburg Y W l a Little Egbert Tract 2084 S o l a n o n p a r C Little Holland Tract 2120 e in e a e M Little Mandeville -

Desilva Island
SUISUN BAY 139 SUISUN BAY 140 SUISUN BAY SUISUN BAY Located immediately downstream of the confluence of the Sacramento and San Joaquin Rivers, Suisun Bay is the largest contiguous wetland area in the San Francisco Bay region. Suisun Bay is a dynamic, transitional zone between the freshwater input of the Central Valley rivers and the tidal influence of the upper San Francisco Estuary. This area supports a substantial number of nesting herons and egrets, including three of the largest colonies in the region. Although suburban development is rampant along the nearby Interstate 80 corridor to the north, most of the Suisun Bay area is protected from heavy development by the California Department of Fish and Game and a number of private duck clubs. Black- Active Great crowned or year Site Blue Great Snowy Night- Cattle last # Colony Site Heron Egret Egret Heron Egret County active Page 501 Bohannon Solano Active 142 502 Campbell Ranch Solano Active 143 503 Cordelia Road Solano 1998 145 504 Gold Hill Solano Active 146 505 Green Valley Road Solano Active 148 506 Hidden Cove Solano Active 149 507 Joice Island Solano 1994 150 508 Joice Island Annex Solano Active 151 509 Sherman Lake Sacramento Active 152 510 Simmons Island Solano 1994 153 511 Spoonbill Solano Active 154 512 Tree Slough Solano Active 155 513 Volanti Solano Active 156 514 Wheeler Island Solano Active 157 SUISUN BAY 141 142 SUISUN BAY Bohannon Great Blue Herons and Great Egrets nest in a grove of eucalyptus trees on a levee in Cross Slough, about 1.8 km east of Beldons Landing. -

Upper Sacramento River Summary Report August 4Th-5Th, 2008
Upper Sacramento River Summary Report August 4th-5th, 2008 California Department of Fish and Game Heritage and Wild Trout Program Prepared by Jeff Weaver and Stephanie Mehalick Introduction: The 400-mile long Sacramento River system is the largest watershed in the state of California, encompassing the McCloud, Pit, American, and Feather Rivers, and numerous smaller tributaries, in total draining nearly one-fifth of the state. From the headwaters downstream to Shasta Lake, referred to hereafter as the Upper Sacramento, this portion of the Sacramento River is a designated Wild Trout Water (with the exception of a small section near the town of Dunsmuir, which is a stocked put-and-take fishery) (Figure 1). The California Department of Fish and Game’s (DFG) Heritage and Wild Trout Program (HWTP) monitors this fishery through voluntary angler survey boxes, creel census, direct observation snorkel surveys, electrofishing surveys, and habitat analyses. The HWTP has repeated sampling over time in a number of sections to obtain trend data related to species composition, trout densities, and habitat condition to aid in the management of this popular sport fishery. In August 2008, the HWTP resurveyed four historic direct observation survey sections on the Upper Sacramento. Methods: Surveys occurred between August 4th and 5th, 2008 with the aid of HWTP personnel and DFG volunteers. Sections were identified prior to the start of the surveys using GPS coordinates, written directions, and past experience. Fish were counted via direct observation snorkel surveys, an effective survey technique in many streams and creeks in California and the Pacific Northwest (Hankin & Reeves, 1988). -

255 Subpart B—First Coast Guard District
SUBCHAPTER G—REGATTAS AND MARINE PARADES PART 100—SAFETY OF LIFE ON 100.703 Special Local Regulations; Recur- ring Marine Events, Sector St. Peters- NAVIGABLE WATERS burg. 100.704 Special Local Regulations; Marine Subpart A—General Events within the Captain of the Port Charleston. Sec. 100.01 Purpose and intent. 100.713 Annual Harborwalk Boat Race; 100.05 Definition of terms used in this part. Sampit River, Georgetown, SC. 100.10 Coast Guard-State agreements. 100.721 Special Local Regulations; Clear- 100.15 Submission of application. water Super Boat National Champion- 100.20 Action on application for event as- ship, Gulf of Mexico; Clearwater Beach, signed to State regulation by Coast FL. Guard-State agreement. 100.724 Annual Augusta Invitational Rowing 100.25 Action on application for event not Regatta; Savannah River, Augusta, GA. assigned to State regulation by Coast 100.732 Annual River Race Augusta; Savan- Guard-State agreement. nah River, Augusta, GA. 100.30 Approval required for holding event. 100.750–100.799 [Reserved] 100.35 Special local regulations. 100.40 Patrol of the regatta or marine pa- Subpart E—Eighth Coast Guard District rade. 100.45 Establishment of aids to navigation. 100.800 [Reserved] 100.50–100.99 [Reserved] 100.801 Annual Marine Events in the Eighth Coast Guard District. Subpart B—First Coast Guard District 100.850–100.899 [Reserved] 100.100 Special Local Regulations; Regattas and Boat Races in the Coast Guard Sec- Subpart F—Ninth Coast Guard District tor Long Island Sound Captain of the 100.900 [Reserved] Port Zone. 100.901 Great Lakes annual marine events. -

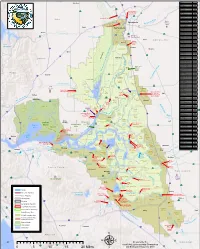
Sacramento River Flood Control System
A p pp pr ro x im a te ly 5 0 M il Sacramento River le es Shasta Dam and Lake ek s rre N Operating Agency: USBR C o rt rr reek th Dam Elevation: 1,077.5 ft llde Cre 70 I E eer GrossMoulton Pool Area: 29,500 Weir ac AB D Gross Pool Capacity: 4,552,000 ac-ft Flood Control System Medford !( OREGON IDAHOIDAHO l l a a n n a a C C !( Redding kk ee PLUMAS CO a e a s rr s u C u s l l Reno s o !( ome o 99 h C AB Th C NEVADA - - ^_ a a Sacramento m TEHAMA CO aa hh ee !( TT San Francisco !( Fresno Las Vegas !( kk ee e e !( rr Bakersfield 5 CC %&'( PACIFIC oo 5 ! Los Angeles cc !( S ii OCEAN a hh c CC r a S to m San Diego on gg !( ny ii en C BB re kk ee ee k t ee Black Butte o rr C Reservoir R i dd 70 v uu Paradise AB Oroville Dam - Lake Oroville Hamilton e M Operating Agency: CA Dept of Water Resources r Dam Elevation: 922 ft City Chico Gross Pool Area: 15,800 ac Gross Pool Capacity: 3,538,000 ac-ft M & T Overflow Area Black Butte Dam and Lake Operating Agency: USACE Dam Elevation: 515 ft Tisdale Weir Gross Pool Area: 4,378 ac 3 B's GrossMoulton Pool Capacity: 136,193Weir ac-ft Overflow Area BUTTE CO New Bullards Bar Dam and Lake Operating Agency: Yuba County Water Agency Dam Elevation: 1965 ft Gross Pool Area: 4,790 ac Goose Lake Gross Pool Capacity: 966,000 ac-ft Overflow Area Lake AB149 kk ee rree Oroville Tisdale Weir C GLENN CO ee tttt uu BB 5 ! Oroville New Bullards Bar Reservoir AB49 ll Moulton Weir aa nn Constructed: 1932 Butte aa CC Length: 500 feet Thermalito Design capacity of weir: 40,000 cfs Design capacity of river d/s of weir: 110,000 cfs Afterbay Moulton Weir e ke rro he 5 C ! Basin e kk Cre 5 ! tt 5 ! u Butte Basin and Butte Sink oncu H Flow from the 3 overflow areas upstream Colusa Weir of the project levees, from Moulton Weir, Constructed: 1933 and from Colusa Weir flows into the Length: 1,650 feet Butte Basin and Sink. -

March 2021 | City of Alameda, California
March 2021 | City of Alameda, California DRAFT ALAMEDA GENERAL PLAN 2040 CONTENTS 04 MARCH 2021 City of Alameda, California MOBILITY ELEMENT 78 01 05 GENERAL PLAN ORGANIZATION + THEMES 6 HOUSING ELEMENT FROM 2014 02 06 LAND USE + CITY DESIGN ELEMENT 22 PARKS + OPEN SPACE ELEMENT 100 03 07 CONSERVATION + CLIMATE ACTION 54 HEALTH + SAFETY ELEMENT 116 ELEMENT MARCH 2021 DRAFT 1 ALAMEDA GENERAL PLAN 2040 ACKNOWLEDGMENTS CITY OF ALAMEDA PLANNING BOARD: PRESIDENT Alan H. Teague VICE PRESIDENT Asheshh Saheba BOARD MEMBERS Xiomara Cisneros Ronald Curtis Hanson Hom Rona Rothenberg Teresa Ruiz POLICY, PUBLIC PARTICIPATION, AND PLANNING CONSULTANTS: Amie MacPhee, AICP, Cultivate, Consulting Planner Sheffield Hale, Cultivate, Consulting Planner Candice Miller, Cultivate, Lead Graphic Designer PHOTOGRAPHY: Amie MacPhee Maurice Ramirez Alain McLaughlin MARCH 2021 DRAFT 3 ALAMEDA GENERAL PLAN 2040 FORWARD Preparation of the Alameda General Plan 2040 began in 2018 and took shape over a three-year period during which time residents, businesses, community groups, and decision-makers reviewed, revised and refined plan goals, policy statements and priorities, and associated recommended actions. In 2020, the Alameda Planning Board held four public forums to review and discuss the draft General Plan. Over 1,500 individuals provided written comments and suggestions for improvements to the draft Plan through the General Plan update website. General Plan 2040 also benefited from recommendations and suggestions from: ≠ Commission on People with Disabilities ≠ Golden -

UC Davis San Francisco Estuary and Watershed Science
UC Davis San Francisco Estuary and Watershed Science Title A Note on Delta Outflow Permalink https://escholarship.org/uc/item/89k7b61m Journal San Francisco Estuary and Watershed Science, 14(3) ISSN 1546-2366 Author Monismith, Stephen G. Publication Date 2016 DOI https://doi.org/10.15447/sfews.2016v14iss3art3 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California OCTOBER 2016 SPECIAL ISSUE: THE STATE OF BAY–DELTA SCIENCE 2016, PART 2 A Note on Delta Outflow Stephen G. Monismith1 Volume 14, Issue 3 | Article 3 KEY WORDS doi: http://dx.doi.org/10.15447/sfews.2016v14iss3art3 1 Department of Civil and Environmental Engineering Sacramento–San Joaquin Delta, San Francisco Bay. Stanford University Suisun Bay, Suisun Marsh, Delta outflow, Dayflow, Stanford, CA 94305 USA tides, salinity, wind stress, hydrodynamics [email protected] INTRODUCTION ABSTRACT The 2013–2015 drought, which has seen California’s water supply storage brought down to historic lows, Outflow from the Sacramento–San Joaquin Delta has focused attention on the management of the flow is a key parameter used in the management of the of water from the Sacramento–San Joaquin Delta San Francisco Bay–Delta system. At present we can (Delta) into San Francisco Bay. This “Delta Outflow” estimate this by assuming a steady state balance is used to maintain desired salinities in Suisun Bay of inflows and outflows (Dayflow) or by direct and Suisun Marsh as well as to keep salinities in the measurement. In this paper, I explore differences Delta sufficiently low to maintain water quality for between observed sub-tidal variations in measured drinking and to irrigate Delta farmlands. -

San Joaquin River Riparian Habitat Below Friant Dam: Preservation and Restoration1
SAN JOAQUIN RIVER RIPARIAN HABITAT BELOW FRIANT DAM: PRESERVATION AND RESTORATION1 Donn Furman2 Abstract: Riparian habitat along California's San Joa- quin River in the 25 miles between Friant Darn and Free- Table 1 – Riparian wildlife/vegetation way 99 occurs on approximately 6 percent of its his- corridor toric range. It is threatened directly and indirectly by Corridor Corridor increased urban encroachment such as residential hous- Category Acres Percent ing, certain recreational uses, sand and gravel extraction, Water 1,088 14.0 aquiculture, and road construction. The San Joaquin Trees 588 7.0 River Committee was formed in 1985 to advocate preser- Shrubs 400 5.0 Other riparianl 1,844 23.0 vation and restoration of riparian habitat. The Com- Sensitive Biotic2 101 1.5 mittee works with local school districts to facilitate use Agriculture 148 2.0 of riverbottom riparian forest areas for outdoor envi- Recreation 309 4.0 ronmental education. We recently formed a land trust Sand and gravel 606 7.5 called the San Joaquin River Parkway and Conservation Riparian buffer 2,846 36.0 Trust to preserve land through acquisition in fee and ne- Total 7,900 100.0 gotiation of conservation easements. Opportunities for 1 Land supporting riparian-type vegetation. In increasing riverbottom riparian habitat are presented by most cases this land has been mined for sand and gravel, and is comprised of lands from which sand and gravel have been extracted. gravel ponds. 2 Range of a Threatened or Endangered plant or animal species. Study Area The majority of the undisturbed riparian habitat lies between Friant Dam and Highway 41 beyond the city limits of Fresno. -

Introduction
INTRODUCTION The purpose of this book is twofold: to provide general information for anyone interested in the California islands and to serve as a field guide for visitors to the islands. The book covers both general history and nat- ural history, from the geological origins of the islands through their aboriginal inhabitants and their marine and terrestrial biotas. Detailed coverage of the flora and fauna of one island alone would completely fill a book of this size; hence only the most common, most readily observed, and most interesting species are included. The names used for the plants and animals discussed in this book are the most up-to-date ones available, based on the scientific literature and the most recently published guidebooks. Common names are always subject to local variations, and they change constantly. Where two names are in common use, they are both mentioned the first time the organism is discussed. Ironically, in recent years scientific names have changed more recently than common names, and the reader concerned about a possible discrepancy in nomenclature should consult the scientific literature. If a significant nomenclatural change has escaped our notice, we apologize. For plants, our primary reference has been The Jepson Manual: Higher Plants of California, edited by James C. Hickman, including the latest lists of errata. Variation from the nomenclature in that volume is due to more recent interpretations, as explained in the text. Certain abbreviations used throughout the text may not be immedi- ately familiar to the general reader; they are as follows: sp., species (sin- gular); spp., species (plural); n. -

San Francisco Bay Plan
San Francisco Bay Plan San Francisco Bay Conservation and Development Commission In memory of Senator J. Eugene McAteer, a leader in efforts to plan for the conservation of San Francisco Bay and the development of its shoreline. Photo Credits: Michael Bry: Inside front cover, facing Part I, facing Part II Richard Persoff: Facing Part III Rondal Partridge: Facing Part V, Inside back cover Mike Schweizer: Page 34 Port of Oakland: Page 11 Port of San Francisco: Page 68 Commission Staff: Facing Part IV, Page 59 Map Source: Tidal features, salt ponds, and other diked areas, derived from the EcoAtlas Version 1.0bc, 1996, San Francisco Estuary Institute. STATE OF CALIFORNIA GRAY DAVIS, Governor SAN FRANCISCO BAY CONSERVATION AND DEVELOPMENT COMMISSION 50 CALIFORNIA STREET, SUITE 2600 SAN FRANCISCO, CALIFORNIA 94111 PHONE: (415) 352-3600 January 2008 To the Citizens of the San Francisco Bay Region and Friends of San Francisco Bay Everywhere: The San Francisco Bay Plan was completed and adopted by the San Francisco Bay Conservation and Development Commission in 1968 and submitted to the California Legislature and Governor in January 1969. The Bay Plan was prepared by the Commission over a three-year period pursuant to the McAteer-Petris Act of 1965 which established the Commission as a temporary agency to prepare an enforceable plan to guide the future protection and use of San Francisco Bay and its shoreline. In 1969, the Legislature acted upon the Commission’s recommendations in the Bay Plan and revised the McAteer-Petris Act by designating the Commission as the agency responsible for maintaining and carrying out the provisions of the Act and the Bay Plan for the protection of the Bay and its great natural resources and the development of the Bay and shore- line to their highest potential with a minimum of Bay fill. -

9.22 Acre Homesite Off of the San Joaquin River of Sherman Island In
Offering Summary Index • A 9.22 +/- acre homesite off of the San Joaquin River of Sherman Island in Cover .................................................................... 1 Sacramento County, CA • The property presents itself as a great potential for a single-family residence. Offering Summary .......................................2 • Located on the scenic San Joaquin River near Highway 160, North of Antioch. Terms, Rights, & Advisories .................. 3 • Reclamation District No. 341 is in the process of doing levee and road Regional Map .................................................4 enhancements near the property. A letter from the Civil Engineers is on file and available for the Buyers to review at their request. Local Area Map ............................................ 5 Location, Legal Description, & Zoning ........................................................... 6 Property Assesment ................................. 6 Purchase Price & Terms ......................... 6 Soils Map ...........................................................7 Slope Map ........................................................ 8 Assessor Map .................................................9 Photos .............................................................. 10 Page 2 All information contained herein or subsequently provided by Broker has been procured directly from the seller or other third parties and has not been verified by Broker. Though believed to be reliable, it is not guaranteed by seller, broker or their agents. Before entering -

A Century of Delta Salt Water Barriers
A Century of Salt Water Barriers in the Delta By Tim Stroshane Policy Analyst Restore the Delta June 5, 2015 edition Since the late 19th century, California’s basic plan for water resource development has been to export water from the Sacramento River and the Delta to the San Joaquin Valley and southern California. Unfortunately, this basic plan ignores the reality that the Delta is the very definition of an estuary: it is where fresh water from the Central Valley’s rivers meets salt water from tidal flow to the Delta from San Francisco Bay. Productive ecosystems have thrived in the Delta for millenia prior to California statehood. But for nearly a century now, engineers and others have frequently referred to the Delta as posing a “salt menace,” a “salinity problem” with just two solutions: either maintain a predetermined stream flow from the Delta to Suisun Bay to hydraulically wall out the tide, or use physical barriers to separate saline from fresh water into the Delta. While readily admitting that the “salt menace” results from reduced inflows from the Delta’s major tributary rivers, the state of California uses salt water barriers as a technological fix to address the symptoms of the salinity problem, rather than the root causes. Given complex Delta geography, these two main solutions led to many proposals to dam up parts of San Francisco Bay, Carquinez Strait, or the waterway between Chipps Island in eastern Suisun Bay and the City of Antioch, or to use large amounts of water—referred to as “carriage water”— to hold the tide literally at bay.