Regulation of Acetylcholinesterase in Neuroblastoma Cells A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Classification of Esterases: an Important Gene Family Involved in Insecticide Resistance - a Review
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 107(4): 437-449, June 2012 437 The classification of esterases: an important gene family involved in insecticide resistance - A Review Isabela Reis Montella1,2, Renata Schama1,2,3/+, Denise Valle1,2,3 1Laboratório de Fisiologia e Controle de Artrópodes Vetores, Instituto Oswaldo Cruz-Fiocruz, Av. Brasil 4365, 21040-900 Rio de Janeiro, RJ, Brasil 2Instituto de Biologia do Exército, Rio de Janeiro, RJ, Brasil 3Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular, Rio de Janeiro, RJ, Brasil The use of chemical insecticides continues to play a major role in the control of disease vector populations, which is leading to the global dissemination of insecticide resistance. A greater capacity to detoxify insecticides, due to an increase in the expression or activity of three major enzyme families, also known as metabolic resistance, is one major resistance mechanisms. The esterase family of enzymes hydrolyse ester bonds, which are present in a wide range of insecticides; therefore, these enzymes may be involved in resistance to the main chemicals employed in control programs. Historically, insecticide resistance has driven research on insect esterases and schemes for their classification. Currently, several different nomenclatures are used to describe the esterases of distinct species and a universal standard classification does not exist. The esterase gene family appears to be rapidly evolving and each insect species has a unique complement of detoxification genes with only a few orthologues across species. The examples listed in this review cover different aspects of their biochemical nature. However, they do not appear to contribute to reliably distinguish among the different resistance mechanisms. -

The Gene for Albicidin Detoxification from Pantoea Dispersa Encodes An
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 9984–9989, September 1997 Plant Biology The gene for albicidin detoxification from Pantoea dispersa encodes an esterase and attenuates pathogenicity of Xanthomonas albilineans to sugarcane (phytotoxin resistanceyleaf scald diseaseyserine hydrolaseypathogenicity factor) LIANHUI ZHANG* AND ROBERT G. BIRCH Department of Botany, The University of Queensland, Brisbane 4072, Australia Communicated by Allen Kerr, University of Adelaide, Adelaide, Australia, June 16, 1997 (received for review April 4, 1997) ABSTRACT Albicidin phytotoxins are pathogenicity fac- produces a family of antibiotics and phytotoxins that block tors in a devastating disease of sugarcane known as leaf scald, DNA replication in bacteria and sugarcane proplastids (13, caused by Xanthomonas albilineans. A gene (albD) from Pantoea 14). The major toxin, named albicidin, has been partially dispersa has been cloned and sequenced and been shown to characterized as a low Mr compound with several aromatic code for a peptide of 235 amino acids that detoxifies albicidin. rings. Because albicidin is rapidly bactericidal to a range of The gene shows no significant homology at the DNA or protein Gram-positive and Gram-negative bacteria at concentrations level to any known sequence, but the gene product contains a as low as 1 ng ml21, it is also of interest as a potential clinical GxSxG motif that is conserved in serine hydrolases. The AlbD antibiotic (15). protein, purified to homogeneity by means of a glutathione Symptoms of leaf scald disease include the emergence of S-transferase gene fusion system, showed strong esterase chlorotic leaves, wilting, necrosis, and sometimes rapid death activity on p-nitrophenyl butyrate and released hydrophilic of plants, often after a prolonged latent period. -

Protein Network Analyses of Pulmonary Endothelial Cells In
www.nature.com/scientificreports OPEN Protein network analyses of pulmonary endothelial cells in chronic thromboembolic pulmonary hypertension Sarath Babu Nukala1,8,9*, Olga Tura‑Ceide3,4,5,9, Giancarlo Aldini1, Valérie F. E. D. Smolders2,3, Isabel Blanco3,4, Victor I. Peinado3,4, Manuel Castell6, Joan Albert Barber3,4, Alessandra Altomare1, Giovanna Baron1, Marina Carini1, Marta Cascante2,7,9 & Alfonsina D’Amato1,9* Chronic thromboembolic pulmonary hypertension (CTEPH) is a vascular disease characterized by the presence of organized thromboembolic material in pulmonary arteries leading to increased vascular resistance, heart failure and death. Dysfunction of endothelial cells is involved in CTEPH. The present study describes for the frst time the molecular processes underlying endothelial dysfunction in the development of the CTEPH. The advanced analytical approach and the protein network analyses of patient derived CTEPH endothelial cells allowed the quantitation of 3258 proteins. The 673 diferentially regulated proteins were associated with functional and disease protein network modules. The protein network analyses resulted in the characterization of dysregulated pathways associated with endothelial dysfunction, such as mitochondrial dysfunction, oxidative phosphorylation, sirtuin signaling, infammatory response, oxidative stress and fatty acid metabolism related pathways. In addition, the quantifcation of advanced oxidation protein products, total protein carbonyl content, and intracellular reactive oxygen species resulted increased -

Reevaluation of Serum Arylesterase Activity in Neurodevelopmental Disorders
antioxidants Article Reevaluation of Serum Arylesterase Activity in Neurodevelopmental Disorders Ignazio Stefano Piras 1,2, Stefano Gabriele 1, Laura Altieri 1, Federica Lombardi 1, Roberto Sacco 1, Carla Lintas 1 , Barbara Manzi 3, Paolo Curatolo 3, Maria Nobile 4, Catia Rigoletto 4, Massimo Molteni 4 and Antonio M. Persico 5,* 1 Unit of Child & Adolescent Neuropsychiatry, University Campus Bio-Medico, I-00128 Rome, Italy; [email protected] (I.S.P.); [email protected] (S.G.); [email protected] (L.A.); [email protected] (F.L.); [email protected] (R.S.); [email protected] (C.L.) 2 Neurogenomics Division, The Translational Genomics Research Institute, Phoenix, AZ 85254, USA 3 Unit of Child and Adolescent Neuropsychiatry, University of Rome “Tor Vergata”, I-00133 Rome, Italy; [email protected] (B.M.); [email protected] (P.C.) 4 Child Psychopathology Unit, Scientific Institute, IRCCS ‘E. Medea’, I-23842 Bosisio Parini (LC), Italy; [email protected] (M.N.); [email protected] (C.R.); [email protected] (M.M.) 5 Interdepartmental Program “Autism 0–90”, “G. Martino” University Hospital, University of Messina, I-98122 Messina, Italy * Correspondence: [email protected] Abstract: Organophosphate compounds (OPs) interfere with neurodevelopment and are neuro- toxic for humans and animals. They are first biotransformed to the more toxic oxon form, and then hydrolyzed to specific metabolites by the enzyme paraoxonase/arylesterase, encoded by the gene PON1 located on human chr. 7q21.3. In autism spectrum disorder (ASD) and in attention- deficit/hyperactivity disorder (ADHD), a correlation between OP exposure and disease onset has Citation: Piras, I.S.; Gabriele, S.; been reported. -

Arylesterase Phenotype-Specific Positive Association Between Arylesterase Activity and Cholinesterase Specific Activity in Human Serum
Int. J. Environ. Res. Public Health 2014, 11, 1422-1443; doi:10.3390/ijerph110201422 OPEN ACCESS International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph Article Arylesterase Phenotype-Specific Positive Association Between Arylesterase Activity and Cholinesterase Specific Activity in Human Serum 1, 2,3 1 Yutaka Aoki *, Kathy J. Helzlsouer and Paul T. Strickland 1 Department of Environmental Health Sciences, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA; E-Mail: [email protected] 2 Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA 3 Mercy Medical Center, Baltimore, MD 21202, USA; E-Mail: [email protected] * Author to whom correspondence should be addressed: E-Mail: [email protected]; Tel.: +1-410-404-9153. Received: 3 June 2013; in revised form: 27 December 2013 / Accepted: 15 January 2014 / Published: 27 January 2014 Abstract: Context: Cholinesterase (ChE) specific activity is the ratio of ChE activity to ChE mass and, as a biomarker of exposure to cholinesterase inhibitors, has a potential advantage over simple ChE activity. Objective: To examine the association of several potential correlates (serum arylesterase/paraoxonase activity, serum albumin, sex, age, month of blood collection, and smoking) with plasma ChE specific activity. Methods: We analyzed data from 195 cancer-free controls from a nested case-control study, accounting for potential confounding. Results: Arylesterase activity had an independent, statistically significant positive association with ChE specific activity, and its magnitude was the greatest for the arylesterase phenotype corresponding to the QQ PON1192 genotype followed by phenotypes corresponding to QR and RR genotypes. -

Serum Paraoxonase and Arylesterase Activities in Metabolic Syndrome In
European Journal of Endocrinology (2011) 164 219–222 ISSN 0804-4643 CLINICAL STUDY Serum paraoxonase and arylesterase activities in metabolic syndrome in Zahedan, southeast Iran Mohammad Hashemi, Dor Mohammad Kordi-Tamandani1, Nooshin Sharifi1, Abdolkarim Moazeni-Roodi2, Mahmoud-Ali Kaykhaei3, Behzad Narouie3 and Adam Torkmanzehi1 Department of Clinical Biochemistry, School of Medicine, Zahedan University of Medical Sciences, Zahedan 98167-43175, Iran, 1Department of Biology, Faculty of Sciences, University of Sistan and Baluchestan, Zahedan 98155-987, Iran and 2Research Center for Infectious Diseases and Tropical Medicine and 3Department of Internal Medicine, School of Medicine, Zahedan University of Medical Sciences, Zahedan 98167-43175, Iran (Correspondence should be addressed to M Hashemi; Email: [email protected]) Abstract Objective: Paraoxonase (PON) is associated with high-density lipoprotein and protects serum lipid from oxidation. The aim of this study was to determine serum PON, arylesterase (ARE) activities, and total antioxidant capacity (TAC) in metabolic syndrome (MES). Methods: This case–control study was performed on 106 patients with MES and 231 healthy subjects. Serum PON and ARE activities were determined spectrophotometrically. TAC was determined using ferric reducing ability of plasma assay. Results: The results showed that serum PON activity was significantly lower in patients with MES (69.62G59.86 IU/l) than healthy subjects (91.64G77.45 IU/l) (P!0.05). The serum ARE activity in MES and normal subjects were 45.23G23.24 and 65.69G31.10 kU/l respectively. The ARE activity was significantly lower in patients with MES than normal subjects (P!0.0001). No significant differences were observed between MES and normal subjects regarding TAC. -
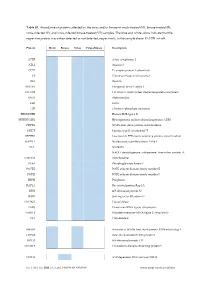
Virus-Infected (V), and Virus-Infected Binase-Treated (VB) Samples
Table S1. Abundance of proteins affected by the virus and/or binase in mock-treated (M), binase-treated (B), virus-infected (V), and virus-infected binase-treated (VB) samples. The blue and white colors indicate that the respective protein was either detected or not detected, respectively, in the sample above 1% FDR cut-off. Protein Mock Binase Virus Virus+Binase Description ACTB Actin, cytoplasmic 1 ATL2 Atlastin-2 CCT7 T-complex protein 1 subunit eta CS Citrate synthase, mitochondrial DES Desmin EEF1A1 Elongation factor 1-alpha 1 EFTUD2 116 kDa U5 small nuclear ribonucleoprotein component ENO1 Alpha-enolase EZR Ezrin GPI Glucose-6-phosphate isomerase HIST1H2BB Histone H2B type 1-B HNRNPA2B1 Heterogeneous nuclear ribonucleoproteins A2/B1 HSPD1 60 kDa heat shock protein, mitochondrial KRT75 Keratin, type II cytoskeletal 75 LRPPRC Leucine-rich PPR motif-containing protein, mitochondrial NAP1L1 Nucleosome assembly protein 1-like 1 NCL Nucleolin NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, NDUFS8 mitochondrial PGK1 Phosphoglycerate kinase 1 POTEE POTE ankyrin domain family member E POTEI POTE ankyrin domain family member I PRPH Peripherin RAP1A Ras-related protein Rap-1A RPS2 40S ribosomal protein S2 SF3B1 Splicing factor 3B subunit 1 TALDO1 Transaldolase TARS Threonine--tRNA ligase, cytoplasmic TARSL2 Probable threonine--tRNA ligase 2, cytoplasmic TKT Transketolase AHSA1 Activator of 90 kDa heat shock protein ATPase homolog 1 HSPA2 Heat shock-related 70 kDa protein 2 RPL15 60S ribosomal protein L15 TXNDC5 Thioredoxin domain-containing protein 5 DDX18 ATP-dependent RNA helicase DDX18 Int. J. Mol. Sci. 2020, 21, x; doi: FOR PEER REVIEW www.mdpi.com/journal/ijms Int. -

Accurate Prediction of Kinase-Substrate Networks Using
bioRxiv preprint doi: https://doi.org/10.1101/865055; this version posted December 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Accurate Prediction of Kinase-Substrate Networks Using Knowledge Graphs V´ıtNov´aˇcek1∗+, Gavin McGauran3, David Matallanas3, Adri´anVallejo Blanco3,4, Piero Conca2, Emir Mu~noz1,2, Luca Costabello2, Kamalesh Kanakaraj1, Zeeshan Nawaz1, Sameh K. Mohamed1, Pierre-Yves Vandenbussche2, Colm Ryan3, Walter Kolch3,5,6, Dirk Fey3,6∗ 1Data Science Institute, National University of Ireland Galway, Ireland 2Fujitsu Ireland Ltd., Co. Dublin, Ireland 3Systems Biology Ireland, University College Dublin, Belfield, Dublin 4, Ireland 4Department of Oncology, Universidad de Navarra, Pamplona, Spain 5Conway Institute of Biomolecular & Biomedical Research, University College Dublin, Belfield, Dublin 4, Ireland 6School of Medicine, University College Dublin, Belfield, Dublin 4, Ireland ∗ Corresponding authors ([email protected], [email protected]). + Lead author. 1 bioRxiv preprint doi: https://doi.org/10.1101/865055; this version posted December 4, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Phosphorylation of specific substrates by protein kinases is a key control mechanism for vital cell-fate decisions and other cellular pro- cesses. However, discovering specific kinase-substrate relationships is time-consuming and often rather serendipitous. -

12) United States Patent (10
US007635572B2 (12) UnitedO States Patent (10) Patent No.: US 7,635,572 B2 Zhou et al. (45) Date of Patent: Dec. 22, 2009 (54) METHODS FOR CONDUCTING ASSAYS FOR 5,506,121 A 4/1996 Skerra et al. ENZYME ACTIVITY ON PROTEIN 5,510,270 A 4/1996 Fodor et al. MICROARRAYS 5,512,492 A 4/1996 Herron et al. 5,516,635 A 5/1996 Ekins et al. (75) Inventors: Fang X. Zhou, New Haven, CT (US); 5,532,128 A 7/1996 Eggers Barry Schweitzer, Cheshire, CT (US) 5,538,897 A 7/1996 Yates, III et al. s s 5,541,070 A 7/1996 Kauvar (73) Assignee: Life Technologies Corporation, .. S.E. al Carlsbad, CA (US) 5,585,069 A 12/1996 Zanzucchi et al. 5,585,639 A 12/1996 Dorsel et al. (*) Notice: Subject to any disclaimer, the term of this 5,593,838 A 1/1997 Zanzucchi et al. patent is extended or adjusted under 35 5,605,662 A 2f1997 Heller et al. U.S.C. 154(b) by 0 days. 5,620,850 A 4/1997 Bamdad et al. 5,624,711 A 4/1997 Sundberg et al. (21) Appl. No.: 10/865,431 5,627,369 A 5/1997 Vestal et al. 5,629,213 A 5/1997 Kornguth et al. (22) Filed: Jun. 9, 2004 (Continued) (65) Prior Publication Data FOREIGN PATENT DOCUMENTS US 2005/O118665 A1 Jun. 2, 2005 EP 596421 10, 1993 EP 0619321 12/1994 (51) Int. Cl. EP O664452 7, 1995 CI2O 1/50 (2006.01) EP O818467 1, 1998 (52) U.S. -

(12) United States Patent (10) Patent No.: US 8,561,811 B2 Bluchel Et Al
USOO8561811 B2 (12) United States Patent (10) Patent No.: US 8,561,811 B2 Bluchel et al. (45) Date of Patent: Oct. 22, 2013 (54) SUBSTRATE FOR IMMOBILIZING (56) References Cited FUNCTIONAL SUBSTANCES AND METHOD FOR PREPARING THE SAME U.S. PATENT DOCUMENTS 3,952,053 A 4, 1976 Brown, Jr. et al. (71) Applicants: Christian Gert Bluchel, Singapore 4.415,663 A 1 1/1983 Symon et al. (SG); Yanmei Wang, Singapore (SG) 4,576,928 A 3, 1986 Tani et al. 4.915,839 A 4, 1990 Marinaccio et al. (72) Inventors: Christian Gert Bluchel, Singapore 6,946,527 B2 9, 2005 Lemke et al. (SG); Yanmei Wang, Singapore (SG) FOREIGN PATENT DOCUMENTS (73) Assignee: Temasek Polytechnic, Singapore (SG) CN 101596422 A 12/2009 JP 2253813 A 10, 1990 (*) Notice: Subject to any disclaimer, the term of this JP 2258006 A 10, 1990 patent is extended or adjusted under 35 WO O2O2585 A2 1, 2002 U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS (21) Appl. No.: 13/837,254 Inaternational Search Report for PCT/SG2011/000069 mailing date (22) Filed: Mar 15, 2013 of Apr. 12, 2011. Suen, Shing-Yi, et al. “Comparison of Ligand Density and Protein (65) Prior Publication Data Adsorption on Dye Affinity Membranes Using Difference Spacer Arms'. Separation Science and Technology, 35:1 (2000), pp. 69-87. US 2013/0210111A1 Aug. 15, 2013 Related U.S. Application Data Primary Examiner — Chester Barry (62) Division of application No. 13/580,055, filed as (74) Attorney, Agent, or Firm — Cantor Colburn LLP application No. -

Novel Protein Pathways in Development and Progression of Pulmonary Sarcoidosis Maneesh Bhargava1*, K
www.nature.com/scientificreports OPEN Novel protein pathways in development and progression of pulmonary sarcoidosis Maneesh Bhargava1*, K. J. Viken1, B. Barkes2, T. J. Grifn3, M. Gillespie2, P. D. Jagtap3, R. Sajulga3, E. J. Peterson4, H. E. Dincer1, L. Li2, C. I. Restrepo2, B. P. O’Connor5, T. E. Fingerlin5, D. M. Perlman1 & L. A. Maier2 Pulmonary involvement occurs in up to 95% of sarcoidosis cases. In this pilot study, we examine lung compartment-specifc protein expression to identify pathways linked to development and progression of pulmonary sarcoidosis. We characterized bronchoalveolar lavage (BAL) cells and fuid (BALF) proteins in recently diagnosed sarcoidosis cases. We identifed 4,306 proteins in BAL cells, of which 272 proteins were diferentially expressed in sarcoidosis compared to controls. These proteins map to novel pathways such as integrin-linked kinase and IL-8 signaling and previously implicated pathways in sarcoidosis, including phagosome maturation, clathrin-mediated endocytic signaling and redox balance. In the BALF, the diferentially expressed proteins map to several pathways identifed in the BAL cells. The diferentially expressed BALF proteins also map to aryl hydrocarbon signaling, communication between innate and adaptive immune response, integrin, PTEN and phospholipase C signaling, serotonin and tryptophan metabolism, autophagy, and B cell receptor signaling. Additional pathways that were diferent between progressive and non-progressive sarcoidosis in the BALF included CD28 signaling and PFKFB4 signaling. Our studies demonstrate the power of contemporary proteomics to reveal novel mechanisms operational in sarcoidosis. Application of our workfows in well-phenotyped large cohorts maybe benefcial to identify biomarkers for diagnosis and prognosis and therapeutically tenable molecular mechanisms. -

Identification of Significant Biomarkers and Pathways Associated with Gastric Carcinogenesis by Whole Genome-Wide Expression Profiling Analysis
INTERNATIONAL JOURNAL OF ONCOLOGY 52: 955-966, 2018 Identification of significant biomarkers and pathways associated with gastric carcinogenesis by whole genome-wide expression profiling analysis HONG-JUN FEI, SONG-CHANG CHEN, JUN-YU ZHANG, SHU-YUAN LI, LAN-LAN ZHANG, YI-YAO CHEN, CHUN-XIN CHANG and CHEN-MING XU Department of Reproductive Genetics, International Peace Maternity and Child Health Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200030, P.R. China Received October 9, 2017; Accepted January 4, 2018 DOI: 10.3892/ijo.2018.4243 Abstract. The incidence of gastric cancer (GC) is extremely of these genes was corroborated in the Oncomine database high in East Asia. GC is also one of the most common and and by Kaplan-Meier plotter (KM-plotter) analysis. Moreover, lethal forms of cancer from a global perspective. However, gastric acid secretion, collecting duct acid secretion, nitrogen to date, we have not been able to determine one or several metabolism and drug metabolism were significantly related to genes as biomarkers in the diagnosis of GC and have also GC. Thus, these genes and pathways may be potential targets been unable to identify the genes which are important in the for improving the diagnosis and clinical effects in patients therapy of GC. In this study, we analyzed all genome-wide with GC. expression profiling arrays uploaded onto the Gene Expression Omnibus (GEO) database to filtrate the differentially Introduction expressed genes (DEGs) between normal stomach tissues and GC tissues. GSE13911, GSE19826 and GSE79973 were Gastric cancer (GC) is the third leading cause of cancer- based on the GPL570 platform, and GSE29272 was based on related mortality in both sexes worldwide (723,000 deaths, the GPL96 platform.