Genetic Evaluation of Carcass Traits in Simmental Cattle by Bruce Cameron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mad-Cow’ Worries Intensify Becoming Prevalent.” Institute
The National Livestock Weekly May 26, 2003 • Vol. 82, No. 32 “The Industry’s Largest Weekly Circulation” www.wlj.net • E-mail: [email protected] • [email protected] • [email protected] A Crow Publication ‘Mad-cow’ worries intensify becoming prevalent.” Institute. “The (import) ban has but because the original diagnosis Canada has a similar feed ban to caused a lot of problems with our was pneumonia, the cow was put Canada what the U.S. has implemented. members and we’re hopeful for this on a lower priority list for testing. Under that ban, ruminant feeds situation to be resolved in very The provincial testing process reports first cannot contain animal proteins be- short order.” showed a possible positive vector North American cause they may contain some brain The infected cow was slaugh- for mad-cow and from there the and spinal cord matter, thought to tered January 31 and condemned cow was sent to a national testing BSE case. carry the prion causing mad-cow from the human food supply be- laboratory for a follow-up test. Fol- disease. cause of symptoms indicative of lowing a positive test there, the Beef Industry officials said due to pneumonia. That was the prima- test was then conducted by a lab Canada’s protocol regarding the ry reason it took so long for the cow in England, where the final de- didn’t enter prevention of mad-cow disease, to be officially diagnosed with BSE. termination is made on all BSE- food chain. they are hopeful this is only an iso- The cow, upon being con- suspect animals. -

Prion Protein Gene (PRNP) Variants and Evidence for Strong Purifying Selection in Functionally Important Regions of Bovine Exon 3
Prion protein gene (PRNP) variants and evidence for strong purifying selection in functionally important regions of bovine exon 3 Christopher M. Seabury†, Rodney L. Honeycutt†‡, Alejandro P. Rooney§, Natalie D. Halbert†, and James N. Derr†¶ †Department of Veterinary Pathobiology, College of Veterinary Medicine, Texas A&M University, College Station, TX 77843-4467; ‡Department of Wildlife and Fisheries Sciences, Texas A&M University, College Station, TX 77843-2258; and §National Center for Agricultural Utilization Research, Agricultural Research Service, U.S. Department of Agriculture, Peoria, IL 61604-3999 Communicated by James E. Womack, Texas A&M University, College Station, TX, September 1, 2004 (received for review December 19, 2003) Amino acid replacements encoded by the prion protein gene indel polymorphism has not been observed within the octapep- (PRNP) have been associated with transmissible and hereditary tide repeat region of ovine PRNP exon 3 (8, 10–20), whereas spongiform encephalopathies in mammalian species. However, an studies of cattle and other bovine species have yielded three indel association between bovine spongiform encephalopathy (BSE) and isoforms possessing five to seven octapeptide repeats (20–31). bovine PRNP exon 3 has not been detected. Moreover, little is Despite the importance of cattle both to agricultural practices currently known regarding the mechanisms of evolution influenc- worldwide and to the global economy, surprisingly little is known ing the bovine PRNP gene. Therefore, in this study we evaluated about PRNP allelic diversity for cattle collectively and͞or how the patterns of nucleotide variation associated with PRNP exon 3 this gene evolves in this lineage. In addition, although several for 36 breeds of domestic cattle and representative samples for 10 nondomesticated species of Bovinae contracted transmissible additional species of Bovinae. -
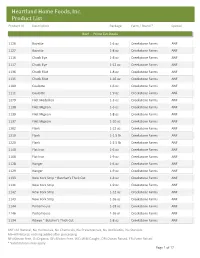
Customer Product List
Heartland Home Foods, Inc. Product List Product Id Description Package Farm / Brand * Special Beef - Prime Cut Steaks 1126 Bavette 1-6 oz Creekstone Farms ANF 1127 Bavette 1-8 oz Creekstone Farms ANF 1116 Chuck Eye 1-8 oz Creekstone Farms ANF 1117 Chuck Eye 1-12 oz Creekstone Farms ANF 1136 Chuck Filet 1-8 oz Creekstone Farms ANF 1135 Chuck Filet 1-16 oz Creekstone Farms ANF 1109 Coulotte 1-6 oz Creekstone Farms ANF 1111 Coulotte 1-9 oz Creekstone Farms ANF 1279 Filet Medallion 1-3 oz Creekstone Farms ANF 1138 Filet Mignon 1-6 oz Creekstone Farms ANF 1139 Filet Mignon 1-8 oz Creekstone Farms ANF 1137 Filet Mignon 1-10 oz Creekstone Farms ANF 1202 Flank 1-12 oz Creekstone Farms ANF 1219 Flank 1-1.5 lb Creekstone Farms ANF 1220 Flank 1-2.5 lb Creekstone Farms ANF 1140 Flat Iron 1-6 oz Creekstone Farms ANF 1168 Flat Iron 1-9 oz Creekstone Farms ANF 1128 Hanger 1-6 oz Creekstone Farms ANF 1129 Hanger 1-9 oz Creekstone Farms ANF 1133 New York Strip ~ Butcher's Thick Cut 1-8 oz Creekstone Farms ANF 1141 New York Strip 1-9 oz Creekstone Farms ANF 1142 New York Strip 1-12 oz Creekstone Farms ANF 1143 New York Strip 1-16 oz Creekstone Farms ANF 1144 Porterhouse 1-18 oz Creekstone Farms ANF 1146 Porterhouse 1-26 oz Creekstone Farms ANF 1134 Ribeye ~ Butcher's Thick Cut 1-8 oz Creekstone Farms ANF ANF=All Natural, No Hormones, No Chemicals, No Preservatives, No Antibiotics, No Steroids AN=All Natural, nothing added after processing NF=Nitrate Free, O=Organic, GF=Gluten Free, WC=Wild Caught, OR=Ocean Raised, FR=Farm Raised * Substitutions may apply -

The Vermont Journal 11-13-19
Rifle PRSRT STD U.S. POSTAGE Season PAID Holiday Happenings POSTAL CUSTOMER RESIDENTIAL CUSTOMER PERMIT #2 Early Holiday Deadlines Opens N. HAVERHILL, NH See Page 3B ECRWSSEDDMECRWSS See Bottom of Page Nov. 16 FREE Your Local Community Newspaper THE NOVEMBERVermont 13, 2019 | WWW.VERMONTJOURNAL.COM JournaVOLUME 19, ISSUEl 46 Area schools welcome Gov. Scott and community to Veterans Day Assembly BY SHARON HUNTLEY to thank someone for their ser- tell his stories and meet gover- The program, celebrating its The Vermont Journal vice. “You should take the time nors. Scott was the 24th gov- seventh year, is due to the vi- to thank a vet or any member ernor that Walton visited. “It sion and hard work of BRHS LUDLOW, Vt. – The seventh of the military, every chance we was a special day for me,” he Booster Club President An- annual Veterans Day Assem- get, every single day,” he said. said. “It’s so important for you, drea Sanford of Ludlow. Ac- bly, Friday, Nov. 8, at Ludlow He then asked all veterans and the younger generation, to do cording to an introduction Elementary School welcomed those serving in the Military to whatever you can to thank our by Color Guard Commander Gov. Phil Scott as part of their stand to be recognized. vets and listen to their stories of American Legion Post 36, moving program to honoring Scott also paid special at- because they truly are heroes Ned Bowen, Sanford went to veterans and active military tention to those of “the great- that set an example for all of the School Board over seven members. -

Richmond 2015-16
RICHMOND StyleWeekly’s Annual Guide to Richmond 2015-16 FIRST PLACE FIRST PLACE W E E E K L L Y Y T ’ S S READERS’ CHOICE 2015 B E D S N T O O M F R I C H THANK John MacLellan Photos & Design & Photos MacLellan John YOU ANNOUNCING OUR 2015-2016 SEASON! BIKINI BABES, SURFER HUNKS AND GIDGET GOES NUTS! THE JOY AND INEVITABILITY OF LIFE, AMIDST GOOD CHICKEN SOUP AND SOME BRISKET PSYCHO BEACH PARTY LAZARUS SYNDROME RICHMOND! by Charles Busch by Bruce Ward; presented as a part of the city-wide Acts of Faith festival JULY 22 – AUGUST 15, 2015 FEBRUARY 24 – MARCH 19, 2016 For voting ABOUT THE PRESSURES OF FAME, PERFECTION AND BEING BARBRA A COMEDY ABOUT EXPLOITATION AND EMPOWERMENT BUYER & CELLAR BODY AWARENESS by Jonathan Tolins by Annie Baker; a co-production with 5th Wall Theatre Project RICHMOND OCTOBER 7–31, 2015 APRIL 20 – MAY 14, 2016 THE KIDS FROM YOUR FAVORITE HOLIDAY STORIES — GROWN-UP, AND SERVED WITH A TWIST! A MUSICAL TRUE STORY, WITH ALL THE GLITTER POSSIBLE TRIANGLE CHRISTMAS ON THE ROCKS THE BOY FROM OZ Conceived by Rob Ruggiero; written by John Cariani, Jeffrey Hatcher, Jacques Lammare, by Martin Sherman and Nick Enright; PLAYERS Matthew Lombardo, Theresa Rebeck, Edwin Sanchez & Jonathan Tolins based on the life and songs of Peter Allen NOVEMBER 18 – DECEMBER 19, 2015 JUNE 8 – JULY 16, 2016 And make sure you check our web site at www.rtriangle.org for our cabaret your favorite nights, special events, and performers checking in from all over the country! theater company! The 2015-16 Season Is Above, some of our Players (left to right): Ian Page, Anna Grey Hogan, Caleb Supported In Part By Funding From Wade, Tarnée Hudson - We 3 Lizas; Danielle Williams, Liz Earnest - 5 Lesbians Eating A Quiche; Audra Honaker - Angels In America; Andrew Etheredge - Pageant; MEDIA SPONSORS: Matt Shofner, Kylie Clark - Angels In America; Steve Boschen - Pageant; Boomie Pederson - Angels In America; (seated) Jeffrey Cole, Matt Polson - Design for 1300 Altamont Avenue Richmond, VA 23230 Living; Drew Colletti, Ed Hughes - YANK!; Jennie Meharg - Design for Living. -

Grass: the Market Potential for U.S. Grassfed Beef 3 Table of Contents
BACK TO GRASS THE MARKET POTENTIAL FOR U.S. GRASSFED BEEF Photo: Carman Ranch ABOUT THIS REPORT Grassfed beef in the U.S. is a fast-growing This report was produced through the consumer phenomenon that is starting to collaboration of Stone Barns Center for Food attract the attention of more cattle producers and Agriculture, a nonprofit sustainable and food companies, but there is a lack of agriculture organization dedicated to changing coherent information on how the market works. the way America eats and farms; Armonia LLC, While the U.S. Department of Agriculture a certified B-Corp with a mission to restore (USDA) produces a vast body of data on the harmony through long-term investments; conventional beef sector, its data collection and Bonterra Partners, an investment consulting reporting efforts on grassfed beef are spotty. firm specializing in sustainable agriculture and Pockets of information are held by different other natural capital investments; and SLM private sector organizations, but they have Partners, an investment management firm that rarely been brought together. focuses on ecological farming systems. The lead authors were Renee Cheung of Bonterra This report addresses that gap by providing Partners and Paul McMahon of SLM Partners; a comprehensive overview of the U.S. they were assisted by Erik Norell, Rosalie Kissel grassfed beef sector, with a focus on market and Donny Benz. and economic dynamics. It brings together available data on the current state of the sector, Dr. Allen Williams of Grass Fed Insights, identifies barriers to growth and highlights LLC acted as a consultant to the project and actions that will help propel further expansion. -

What Have We Learned from Cattle/Beef Disputes?
What Have We Learned from Cattle/Beef Disputes? R.M.A. Loyns, President, Prairie Horizons, Ltd. and Former Professor, University of Manitoba; Linda M. Young, Agricultural Policy Coordinator, Trade Research Center; and Colin A. Carter, Professor, University of California–Davis Research Discussion Paper No. 41 April 2000 The purpose of research discussion papers is to make research findings available to researchers and the public before they are available in professional journals. Consequently, they are not peer reviewed. This paper was first prepared for presentation at a conference, Trade Liberalization under NAFTA: Report Card in Agriculture, sponsored by the Policy Disputes Information Consortium. The Consortium, with members from NAFTA countries representing government, business, and academia, have sponsored six workshops on agricultural policy issues within NAFTA. Their publications are discussed at www.farmfoundation.org/pubs2.htm. The authors acknowledge research/drafting assistance provided by Kitty Sue Squires (MSU), Julia Davis (UC Davis), and the NCBA and CCA for providing documents for review. The authors also want to thank Gary Brester and John Marsh (MSU) for the generous use of several graphs. What Have We Learned from Cattle/Beef Disputes? Abuse of important trade laws represents one of the most ominous threats to a liberal international trading regime. Joseph Stiglitz, SEJ, 1997. BACKGROUND AND PURPOSE OF THE PAPER In this paper, informal and formal disputes in the cattle/beef sector are identified because they are both important to understanding trading relations among the United States, Mexico, and Canada. R-CALF, and antidumping duties imposed by Mexico against imports of U.S. beef in 1999, are the only formal disputes that we have found. -

Nutribalance-5000 Nutritional Scale
NutriBalance-5000 Nutritional Scale Carb. Guide Contains over 7000 additional food codes for carbohydrates! oz Max: 11lb d: 0.1oz MR M+ WT 9 Prot 7 8 Cal Sal 0 Tare 6 Fat Carb Col 4 5 Fibr 3 g/oz CLR 2 WT MC 1 How To Use This Manual: This manual provides a cross-reference of carbohydrate codes for the NutriBalance nutritional scale, based on the USDA National Nutrient Database Release 18. When using this manual, only the Carb function of the Nutribalance should be used. All other nutritional buttons such as Fiber, Prot, etc will not display accurate information. 1. To find the Carb Code for a food item, simply use the Acrobat Search function (Ctrl+F or Ctrl+Shift+F). Enter the name of the food item in the Search Field and hit Enter. Give the search time to complete. 2. Once you find your food item in the manual, select your code from the “Code to use” column, or the Code (Fiber Method) column. 3. Place the food item onto the weighing platform and enter the code using the keypad. Now press the Carb button. NOTE: The NutriBalance requires 3-digit input for the code to be accepted. Therefore, if the “Code to use” is 3, you should enter 003, etc. Code to use Code Carbo- Fiber_ Refuse_ Modified ( Fiber hydrt TD Pct Carbs (- Method) fiber) MILK SUBSTITUTES,FLUID,W/ 41 41 6.16 0 0 6.16 LAURIC ACID OIL MILK,WHL,3.25% MILKFAT 85 85 4.52 0 0 4.52 MILK,PRODUCER,FLUID,3.7% 819 819 4.65 0 0 4.65 MILKFAT MILK,RED 819 819 4.68 0 0 4.68 FAT,FLUID,2%MILKFAT,W/ADDED VIT A MILK,RED FAT,FLUID,2% 696 696 4.97 0 0 4.97 MILKFAT,W/ NONFAT MILK SOL&VIT A MILK,RED -

Cattle Markets Task Force REPORT
Cattle Markets Task Force REPORT SEPTEMBER 2020 TABLE OF CONTENTS I. Executive Summary Page 3 II. Cattle Markets Task Force Report Page 5 III. The Work of the Task Force Page 6 IV. Nebraska’s Cattle Industry Page 10 V. Task Force Recommendations Pages 16-33 V. i - Fed Cattle Markets Page 16 V. ii - Livestock Market Reporting Act Page 21 V. iii - Small and Medium-Sized Packing Facilities Page 23 V. iv - Packer Market Power Page 25 V. v - Risk Management Page 30 V. vi - Mandatory Country of Origin Labeling (MCOOL) Page 33 VI. Conclusion Page 36 2 Cattle Markets Task Force Report - September 2020 I. Executive Summary SUMMARY OF TASK FORCE RECOMMENDATIONS Following the large cattle market and boxed beef price shifts after the fire at the Tyson beef processing facility in Holcomb, Kansas, the Nebraska Farm Bureau (NEFB) State Board of Directors voted to create a Cattle Markets Task Force charged with examining current Farm Bureau policy, providing policy recommendations, and providing input on what NEFB’s role should be in addressing concerns regarding cattle markets. The closure of a number of processing facilities due to the COVID-19 pandemic created an even larger disparity between the price producers received vs. retail and boxed beef prices. Over the course of five months, the NEFB Cattle Markets Task Force met online and in person with agriculture economists, cattle organizations, auction barn owners, feedlot managers, restaurant owners, and consultants in order to gain a better understanding of the entire beef supply chain. Following the Task Force’s initial meetings, the group decided on six topics to explore and ultimately suggest policy resolutions. -

The Main Beef Breeds
MODULE 1A PART D: STUDENT FACTSHEET - THE MAIN BEEF BREEDS Breed Characteristics Dam Mature Sire Mature Origin Purpose Weight Weight • Colour black or red • Developed in Scotland from cattle native to Aberdeen Angus Aberdeenshire and Angus 600-900kg 900-1100kg Scotland Terminal sire • Naturally polled • Popular beef breed in tHe United States • A fertile cross between domesticated cattle (Bos Taurus) and American Bison (Bison bison). THe intention of tHe cross is to combine tHe lower fat and cholesterol, cold Beefalo resistance and easy calving qualities of the variable variable UDA Cig Eidion Bison with the docility and higher growth rates of domestic cattle. It is only a Beefalo if the cross is 5/8 ths (37.5%) Bison, if the proportion is higher then its called a Hybrid Bison. • Colour varies from white to black. • Large long body witH double muscle in Hind quarter. British Belgian Blues 800kg 1300kg Terminal sire • HigH saleable meat yield Belgium • Increased dystocia an issue witH tHe breed 1 Breed Characteristics Dam Mature Sire Mature Origin Purpose Weight Weight • White-Tan colour • Ease of calving • HigH growtH rates British Blonde 600 kg 700-1100kg France Terminal sire • Unbroken wHeat coloured • Extended gestation period • Colour creamy wHite througH to wHeat • First continental breed of cattle to be introduced to Great Britain • Initial importation of bulls by dairy Terminal sire and sire for ¾ Charolais 600-900kg 900-1100kg France producers seeking a sire to improve their continental suckler cows. calves’ conformation. • HigH daily liveweigHt gain and improved conformation. • Hardy suckler cow breed Galloway • Suitable for low input systems 400 – 600kg 800 - 950 kg Scotland Native suckler cow breed • Long lived cows • White face and red coat • Easy temperament Hereford 700 – 800kg 1200 – 1500kg Hereford Terminal sire • Main terminal sire breed prior to importation of continental breeds. -

MICHIGAN BEEF PRODUCTION COOPERATIVE EXTENSION SERVICE • MICHIGAN STATE UNIVERSITY Selecting a Breed of Beef Cattle Harlan D
Extension Bulletin E-1755 February 1984 (NEW) 80 cents MICHIGAN BEEF PRODUCTION COOPERATIVE EXTENSION SERVICE • MICHIGAN STATE UNIVERSITY Selecting a Breed of Beef Cattle Harlan D. Ritchie Department of Animal Science Criteria For Choosing A Breed considered good milkers. Angus females are known Selecting a breed or combination of breeds to use for their fertility and ease of calving. The breed is in your beef herd should be based on the following nearly pure for the polled trait and Angus bulls can be criteria: (1) marketability in your area; (2) cost and expected to sire calf crops that are 100% hornless. availability of good seedstock; (3) climate; (4) quantity The dark skin pigment provides some resistance and quality of feedstuffs on your farm; (5) how the against cancer eye and sun-burned udders. breeds used in a crossing program complement one Angus calves fatten quickly and grade Choice at a another; and (6) personal preference. As an example of relatively light weight (1,050 lb.). They possess more climatic adaptability, British breeds are well adapted marbling in the meat than any other breed of cattle, to cold climates, but do not fare as well in sub which means their quality grade (Prime, Choice, tropical regions. Conversely, Brahman blood is need Good, etc.) is often higher than that of other cattle. ed for optimum performance in certain Gulf Coastal For this reason, some packers pay a premium for areas, but is not required in the northern states. Angus or Angus-cross steers. However, feedlot operators sometimes pay less for Angus feeder calves British Breeds because they have a tendency to mature too quickly and become fat at too light a weight. -

Troubling Species
TROUBLING SPECIES Care and Belonging in a Relational World THE MULTISPECIES EDITING COLLECTIVE Transformations in Environment and Society 2017 / 1 RCC Perspectives: Transformations in Environment and Society is an open-access publication that exists to record and reflect the activities of the Rachel Carson Center for Environment and Society. The journal provides a forum for examining the interrelationship between environmen- tal and social changes and is designed to inspire new perspectives on humanity and the wider world. RCC Perspectives aims to bridge the gap between scholarly and non-scholarly audiences and encourage international dialogue. All issues of RCC Perspectives are available online. To view past issues, please visit www.environmentandsociety.org/perspectives. Troubling Species Care and Belonging in a Relational World THE MULTISPECIES EDITING COLLECTIVE RCC Perspectives Transformations in Environment and Society 2017 / 1 Troubling Species 3 Contents 5 Introduction Etienne S. Benson, Veit Braun, Jean M. Langford, Daniel Münster, Ursula Münster, and Susanne Schmitt, with the support of the Multispecies Editing Collective Multispecies Belonging 11 When Species Fall Apart Jean M. Langford 19 The Domestic Stain, or Maintaining Standards Harriet Ritvo 25 Zero Budget Natural Farming and Bovine Entanglements in South India Daniel Münster 33 Caring, Hating, and Domesticating: Bird Protection and Cats in Imperial Germany Amir Zelinger 41 Pelicans: Protection, Pests, and Private Property Emily O’Gorman 49 The Cattle Guard Etienne S. Benson Multispecies Care 59 Making Worlds with Crows: Philosophy in the Field Thom van Dooren 67 The Sons of Salim Ali: Avian Care in the Western Ghats of South India Ursula Münster 77 Interspecies Care in a Hybrid Institution Piers Locke 83 Care, Gender, and Survival: The Curious Case of the Seahorse Susanne Schmitt 91 Viral Ethnography: Metaphors for Writing Life Celia Lowe 97 Of Mice and Men: Ecologies of Care in a Climate Chamber Veit Braun Troubling Species 5 Etienne S.