Allergic Conjunctivitis Leonard Bielory, Mda, Mitchell H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Differentiate Red Eye Disorders
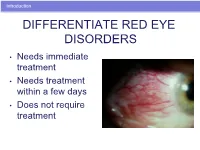
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

A Description of the Clinical Features of Brimonidine- Associated Uveitis Alyssa Louie Primary Care Resident, San Francisco VA
Drug-induced intraocular inflammation: A description of the clinical features of brimonidine- associated uveitis Alyssa Louie Primary Care Resident, San Francisco VA Abstract: A description of the clinical features, diagnostic work-up, and management of acute anterior uveitis caused by brimonidine, a widely used glaucoma medication. I. Case History a. Patient demographics: 74 year-old white male b. Chief complaint: eye pain, redness, irritation for last 2 weeks c. Ocular and medical history: i. Ocular history 1. Primary open angle glaucoma OU, diagnosed 8 years ago 2. Senile cataracts OU, not visually significant 3. Type 2 Diabetes without retinopathy OU 4. No prior history of uveitis ii. Medical history: Diabetes Mellitus Type 2 iii. No known drug allergies d. Medications i. Ocular: dorzolamide BID OU (1.5 years), brimonidine BID OU (11 months), travatan QHS OU (5.5 years) ii. Medical: metformin 500mg tab BID PO II. Pertinent Findings a. Clinical exam i. Visual acuities: OD 20/20-, OS 20/20- ii. Goldmann applanation tonometry: 13 mm Hg OD, 13 mm Hg OS iii. Anterior segment 1. OU: 3+ diffuse conjunctival injection 2. OU: central and inferior granulomatous keratic precipitates 3. OU: Grade 1+ cell, 1+ flare 4. OU: No synechiae or iris changes were present iv. Posterior segment 1. Optic Nerve a. OD: Cup-to-disc ratio 0.70H/V, distinct margins b. OS: Cup-to-disc ratio 0.75H/V, distinct margins 2. Posterior pole, periphery, vitreous: unremarkable OU b. Laboratory Studies i. ACE, Lysozyme, FTA-ABS, VDRL, HLA-B27, Rheumatoid Factor, ANA, PPD, Chest X- ray: all negative/unreactive III. -

Treatment of Allergic Conjunctivitis with Olopatadine Hydrochloride Eye Drops
REVIEW Treatment of allergic conjunctivitis with olopatadine hydrochloride eye drops Eiichi Uchio Abstract: Olopatadine hydrochloride exerts a wide range of pharmacological actions such as histamine H receptor antagonist action, chemical mediator suppressive action, and eosinophil Department of Ophthalmology, 1 Fukuoka University School of infi ltration suppressive action. Olopatadine hydrochloride 0.1% ophthalmic solution (Patanol®) Medicine, Fukuoka, Japan was introduced to the market in Japan in October 2006. In a conjunctival allergen challenge (CAC) test, olopatadine hydrochloride 0.1% ophthalmic solution signifi cantly suppressed ocular itching and hyperemia compared with levocabastine hydrochloride 0.05% ophthalmic solution, and the number of patients who complained of ocular discomfort was lower in the olopatadine group than in the levocabastine group. Conjunctival cell membrane disruption was observed in vitro in the ketotifen fumarate group, epinastine hydrochloride group, and azelastine hydrochloride group, but not in the olopatadine hydrochloride 0.1% ophthalmic solution group, which may potentially explain the lower discomfort felt by patients on instillation. Many other studies in humans have revealed the superiority of olopatadine 0.1% hydrochloride eye drops to several other anti-allergic eye drops. Overseas, olopatadine hydrochloride 0.2% ophthalmic solution for a once-daily regimen has been marketed under the brand name of Pataday®. It is expected that olopatadine hydrochloride ophthalmic solutions may be used in patients with a more severe spectrum of allergic conjunctival diseases, such as vernal keratoconjunctivitis or atopic keratoconjunctivitis, in the near future. Keywords: olopatadine, eye drop, allergic conjunctivitis, anti-histaminergic Introduction The prevalence of allergic conjunctival diseases (ACD) in Japan is estimated to be as high as 15%–20% of the population and is on the rise. -

Ophthalmic Antihistamines
Ophthalmics for Allergic Conjunctivitis Review 04/12/2011 Copyright © 2004 - 2011 by Provider Synergies, L.L.C. All rights reserved. Printed in the United States of America. All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage and retrieval system without the express written consent of Provider Synergies, L.L.C. All requests for permission should be mailed to: Attention: Copyright Administrator Intellectual Property Department Provider Synergies, L.L.C. 10101 Alliance Rd, Ste 201 Cincinnati, Ohio 45242 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, -

Characteristics of Allergy in Autoimmune Thyroid Diseases Ildikó
Characteristics of allergy in autoimmune thyroid diseases Ildikó Molnár MD, PhD, EndoMed, Hungary ImmunSum, Baltimore, 2014 Relationship between allergic responses and thyroid autoimmunity IgE levels IgE deposits are present in Graves’ thyroid and orbital tissues (Werner SC et al., N Engl Med, 1972;287:421-425.; Raikow RB et al., Ophthalmol. 1990; 97:629-635.) Elevated IgE levels associated with hyperthyroid Graves’ disease (Akira S et al., J Clin Endocrinol Metab 1999; 84:3602-3605.; Takashi Y et al., J Clin Endocrinol Metab 2000; 85:2775- 2778.) Evidence of immunglobulin E autoantibodies to thyrotropin receptor (TSH rec) and thyroid peroxidase (TPO) (Metcalfe R et al., J Clin Endocrinol Metab 2002;87:1754-1761.; Gou J et al., Clin Immunol Immunopathol 1997; 82: 157-162.) Th2-derived cytokine profils Elevated serum levels of IL-5 and IL-13 cytokines. (Hidaka Y et al., Thyroid 1998; 8:235-239.; Ichiro K et al., J Clin Endocrinol Metab 2001; 86:3540-3544.) Allergic rhinitis associated frequently with Graves’ disease (Amino N et al., Thyroid 2003; 13:811-814.; Hidaka Y et al., Thyroid 1996; 6: 349-351.) Common key factors regulate the immune responses in both allergic and autoimmune conditions (Rottem M et al., Dev Immunology 2002; 9: 161-167.) ImmunSum, Baltimore, 2014 Previous results Graves’ ophthalmopathy associated with increased total IgE serum levels. Molnár I et al., Eur J Med Rev 1996; 1:543-546. Hyperthyroid Graves’ ophthalmopathy demonstrated elevated serum IL-5 levels compared to patients who had no eye signs. Molnár I , Abstract: ACT International Suppl., 2000; 2: 220. Decreased serum levels of nerve growth factor (NGF) associated with hyperthyroid Graves’ ophthalmopathy compared to those who had no eye signs. -

Pediatric Pharmacology and Pathology
7/31/2017 In the next 2 hours……. Pediatric Pharmacology and Pathology . Ocular Medications and Children The content of th is COPE Accredited CE activity was prepared independently by Valerie M. Kattouf O.D. without input from members of the optometric community . Brief review of examination techniques/modifications for children The content and format of this course is presented without commercial bias and does not claim superiority of any commercial product or service . Common Presentations of Pediatric Pathology Valerie M. Kattouf O.D., F.A.A.O. Illinois College of Optometry Chief, Pediatric Binocular Vision Service Associate Professor Ocular Medications & Children Ocular Medications & Children . Pediatric systems differ in: . The rules: – drug excretion – birth 2 years old = 1/2 dose kidney is the main site of drug excretion – 2-3 years old = 2/3 dose diminished 2° renal immaturity – > 3 years old = adult dose – biotransformation liver is organ for drug metabolism Impaired 2° enzyme immaturity . If only 50 % is absorbed may be 10x maximum dosage Punctal Occlusion for 3-4 minutes ↓ systemic absorption by 40% Ocular Medications & Children Ocular Medications & Children . Systemic absorption occurs through….. Ocular Meds with strongest potential for pediatric SE : – Mucous membrane of Nasolacrimal Duct 80% of each gtt passing through NLD system is available for rapid systemic absorption by the nasal mucosa – 10 % Phenylephrine – Conjunctiva – Oropharynx – 2 % Epinephrine – Digestive system (if swallowed) Modified by variation in Gastric pH, delayed gastric emptying & intestinal mobility – 1 % Atropine – Skin (2° overflow from conjunctival sac) Greatest in infants – 2 % Cyclopentalate Blood volume of neonate 1/20 adult Therefore absorbed meds are more concentrated at this age – 1 % Prednisone 1 7/31/2017 Ocular Medications & Children Ocular Medications & Children . -

The Role of Eosinophils and Basophils in Allergic Diseases Considering Genetic Findings
The role of eosinophils and basophils in allergic diseases considering genetic findings. Rachel Nadif, Farid Zerimech, Emmanuelle Bouzigon, Regis Matran To cite this version: Rachel Nadif, Farid Zerimech, Emmanuelle Bouzigon, Regis Matran. The role of eosinophils and ba- sophils in allergic diseases considering genetic findings.. Current Opinion in Allergy and Clinical Im- munology, Lippincott, Williams & Wilkins, 2013, 13 (5), pp.507-13. 10.1097/ACI.0b013e328364e9c0. inserm-00880260 HAL Id: inserm-00880260 https://www.hal.inserm.fr/inserm-00880260 Submitted on 3 Oct 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The role of eosinophils and basophils in allergic diseases considering genetic findings Rachel Nadifa,b, Farid Zerimechc,d, Emmanuelle Bouzigone,f, Regis Matranc,d Affiliations: aInserm, Centre for research in Epidemiology and Population Health (CESP), U1018, Respiratory and Environmental Epidemiology Team, F-94807, Villejuif, France bUniv Paris-Sud, UMRS 1018, F-94807, Villejuif, France cCHRU de Lille, F-59000, Lille, France dUniv Lille Nord de France, EA4483, F-59000, Lille, France eUniv Paris Diderot, Sorbonne Paris Cité, Institut Universitaire d’Hématologie, F-75007, Paris, France fInserm, UMR-946, F-75010, Paris, France Correspondence to Rachel Nadif, PhD, Inserm, Centre for research in Epidemiology and Population Health (CESP), U1018, Respiratory and Environmental Epidemiology Team, F-94807, Villejuif, France. -

Food Allergy and Intolerance: a Narrative Review on Nutritional Concerns
nutrients Review Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns Domenico Gargano 1,†, Ramapraba Appanna 2,†, Antonella Santonicola 2 , Fabio De Bartolomeis 1, Cristiana Stellato 2, Antonella Cianferoni 3, Vincenzo Casolaro 2 and Paola Iovino 2,* 1 Allergy and Clinical Immunology Unit, San Giuseppe Moscati Hospital, 83100 Avellino, Italy; [email protected] (D.G.); [email protected] (F.D.B.) 2 Department of Medicine, Surgery and Dentistry “Scuola Medica Salernitana”, University of Salerno, 84081 Baronissi, Italy; [email protected] (R.A.); [email protected] (A.S.); [email protected] (C.S.); [email protected] (V.C.) 3 Division of Allergy and Immunology, The Children’s Hospital of Philadelphia, Perelman School of Medicine at University of Pennsylvania, Philadelphia, PA 19104, USA; [email protected] * Correspondence: [email protected]; Tel.: +39-335-7822672 † These authors contributed equally to this work. Abstract: Adverse food reactions include immune-mediated food allergies and non-immune-mediated intolerances. However, this distinction and the involvement of different pathogenetic mechanisms are often confused. Furthermore, there is a discrepancy between the perceived vs. actual prevalence of immune-mediated food allergies and non-immune reactions to food that are extremely common. The risk of an inappropriate approach to their correct identification can lead to inappropriate diets with severe nutritional deficiencies. This narrative review provides an outline of the pathophysiologic and clinical features of immune and non-immune adverse reactions to food—along with general Citation: Gargano, D.; Appanna, R.; diagnostic and therapeutic strategies. Special emphasis is placed on specific nutritional concerns for Santonicola, A.; De Bartolomeis, F.; each of these conditions from the combined point of view of gastroenterology and immunology, in Stellato, C.; Cianferoni, A.; Casolaro, an attempt to offer a useful tool to practicing physicians in discriminating these diverging disease V.; Iovino, P. -

What Is Allergy? Allergies Are Increasing in Australia and New Zealand and Affect Around One in Five People
ASCIA INFORMATION FOR PATIENTS, CONSUMERS AND CARERS What is Allergy? Allergies are increasing in Australia and New Zealand and affect around one in five people. There are many causes of allergy, and symptoms vary from mild to potentially life threatening. Allergy is one of the major factors associated with the cause and persistence of asthma. The definition of allergy Allergy occurs when a person's immune system reacts to substances in the environment that are harmless to most people. These substances are known as allergens and are found in dust mites, pets, pollen, insects, ticks, moulds, foods and some medications. Atopy is the genetic tendency to develop allergic diseases. When atopic people are exposed to allergens, they can develop an immune reaction that leads to allergic inflammation. This can cause symptoms in the: • Nose and/or eyes, resulting in allergic rhinitis (hay fever) and/or conjunctivitis. • Skin resulting in eczema, or hives (urticaria). • Lungs resulting in asthma. What happens when you have an allergic reaction? When a person who is allergic to a particular allergen comes into contact with it, an allergic reaction occurs: • When the allergen (such as pollen) enters the body it triggers an antibody response. • The antibodies attach themselves to mast cells. • When the pollen comes into contact with the antibodies, the mast cells respond by releasing histamine. • When the release of histamine is due to an allergen, the resulting inflammation (redness and swelling) is irritating and uncomfortable. Similar reactions can occur to some chemicals and food additives. However, if they do not involve the immune system, they are known as adverse reactions, not allergy. -

Association of Pediatric Atopic Dermatitis and Cataract Development and Surgery
Supplementary Online Content Jeon HS, Choi M, Byun SJ, Hyon JY, Park KH, Park SJ. Association of pediatric atopic dermatitis and cataract development and surgery. JAMA Ophthamol. Published online June 7, 2018. doi:10.1001/jamaophthalmol.2018.2166 eTable 1. List of diagnostic codes used for defining subjects eTable 2. Drug lists used for defining atopic dermatitis eTable 3. Baseline characteristics of atopic dermatitis cohort and control group eTable 4. Cox analysis-derived hazard ratios (HRs) and 95% confidence intervals (CIs) of cataract development associated with atopic dermatitis (AD) and its covariates eTable 5. Cox analysis-derived hazard ratios (HRs) and 95% confidence intervals (CIs) of cataract surgery associated with atopic dermatitis (AD) and its covariates This supplementary material has been provided by the authors to give readers additional information about their work. © 2018 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 09/24/2021 eTable 1. List of diagnostic codes used for defining subjects Diagnoses Code numbers in KCD-6a Atopic dermatitis L20 Cataract H25, H26 Congenital malformation of eyes (anopthalmos, microphthalmos, and Q11 macrophthalmos) Congenital lens malformation Q12 Congenital malformation of anterior segment of eyes Q13 Congenital malformation of posterior segment of eyes Q14 Other congenital malformations of eyes Q15 Cataract surgery S5110, S5111, S5112, S5119 Asthma J45.0, J45.9 Allergic rhinitis J30.1, J30.2, J30.3, J30.4 aThe diagnosis was coded according to the Korean Classification of Disease, 6th edition (KCD-6, a version of the International Classification of Diseases, 10th edition, adapted for the Korean healthcare system). © 2018 American Medical Association. -

ยากลุ่ม Ophthalmic Anti-Allergics No. ชื่อยา รูปแบบ สรุปเหตุผลกา 1
ยากลุ่ม Ophthalmic Anti-allergics No. ชื่อยา รูปแบบ สรุปเหตุผลการเลือกยา 1 Sodium cromoglicate eye drop บัญชี ค (Disodium เงื่อนไข (ไม่ระบุ) Cromoglycate /Cromolyn sodium ) 2 Lodoxamide eye drop ไม่คัดเลือกไว้ในบัญชี trometramine เหตุผล ไม่คัดเลือกตามบัญชียาหลัก พศ.2551 3 Naphazoline HCl eye drop ไม่คัดเลือกไว้ในบัญชี เหตุผล ไม่คัดเลือกตามบัญชียาหลัก พศ.2551 4 Naphazoline HCl+ eye drop และไม่ผ่าน ISafE score Pheniramine maleate 5 Naphazoline nitrate + eye drop zinc sulfate 6 Olopatadine eye drop ไม่คัดเลือกไว้ในบัญชี hydrochloride เหตุผล ไม่มีข้อมูลหลักฐานที่ชัดเจนว่ามีประสิทธิภาพ 7 Ketotifen fumarate eye drop หรือความปลอดภัยเหนือกว่ายา Sodium cromoglicate 8 Antazoline HCl eye drop บัญชี ก +Tetrahydrozoline HCl เงื่อนไข (ไม่ระบุ) 9 Tetrahydrozoline HCl eye drop ไม่คัดเลือกไว้ในบัญชี เหตุผล ไม่คัดเลือกตามบัญชียาหลัก พ.ศ.2551 10 Epinastine HCl eye drop และไม่ผ่าน ISafE score 11 Pemirolast potassium eye drop 12 Emedastine fumarate eye drop ไม่คัดเลือกไว้ในบัญชี เหตุผล ไม่คัดเลือกตามบัญชียาหลัก พ.ศ.2551 และผู้ผลิตได้ยกเลิกทะเบียนแล้ว ส่วนท่ ี 1 ข้อมูลโดยสรุป กลุ่มยาทางเลือกแรกในการรักษาอาการเยื่อบุตาอักเสบจากการแพ้ ได้แก่ topical antihistamines และ mast cell stabilizers ยาทางเลือกอื่นเป็นสูตรผสม separate topical mast cell stabilizer และ topical antihistamine ได้แก่ olopatadine, azelastine HCl, epinastine,pemirolast potassium, และ ketotifen fumarate ข้อมูลด้านประสิทธิผลพบว่า US.FDA อนุมัติ Olopatadine ข้อบ่งใช้ Allergic conjunctivitis ในผู้ใหญ่และ เด็กอายุ 3 ปีขึ้นไป, Ketotifen ข้อบ่งใช้ Allergic conjunctivitis - Itching; Prophylaxis ในผู้ใหญ่และเด็กอายุ -

USP Medicare Model Guidelines V6.0 Page 1 of 56
USP Medicare Model Guidelines v6.0 Page 1 of 56 ABCDE Example Part D USP Category USP Class Salt/Ester Change language 2 Eligible Drugs* 3 Analgesics Nonsteroidal Anti-inflammatory 4 Drugs 5 Celecoxib 6 Diclofenac Potassium 7 Diflunisal 8 Etodolac 9 Fenoprofen Calcium 10 Flurbiprofen 11 Ibuprofen 12 Indomethacin 13 Ketoprofen 14 Ketorolac Tromethamine 15 Meclofenamate 16 Mefenamic Acid 17 Meloxicam 18 Nabumetone 19 Naproxen 20 Oxaprozin 21 Piroxicam 22 Sulindac 23 Tolmetin 24 Opioid Analgesics, Long-acting 25 Hydromorphone 26 Fentanyl 27 Levorphanol Tartrate 28 Methadone Hydrochloride 29 Morphine Sulfate 30 Oxycodone Hydrochloride 31 Oxymorphone Hydrochloride 32 Tramadol Hydrochloride 33 Opioid Analgesics, Short-acting 34 Butorphanol Tartrate 35 Codeine Phosphate 36 Fentanyl Citrate 37 Hydromorphone Hydrochloride * This list is illustrative of Part D eligible drugs only, and does not infer CMS coverage Changes from USP MMGv5.0 marked in red USP Medicare Model Guidelines v6.0 Page 2 of 56 ABCDE Example Part D USP Category USP Class Salt/Ester Change language 2 Eligible Drugs* 38 Meperidine Hydrochloride 39 Nalbuphine Hydrochloride 40 Oxycodone Hydrochloride 41 Oxymorphone Hydrochloride 42 Pentazocine Lactate 43 Tapentadol 44 Tramadol Hydrochloride 45 Anesthetics 46 Local Anesthetics 47 Lidocaine Hydrochloride 48 Lidocaine and Prilocaine Anti-Addiction/ Substance Abuse Treatment Agents 49 50 Alcohol Deterrents/Anti-craving 51 Acamprosate Calcium 52 Disulfiram 53 Naltrexone Hydrochloride Nomenclature change; New Class Opioid Dependence Treatments