Ocular Allergy As a Risk Factor for Dry Eye in Adults and Children
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differentiate Red Eye Disorders
Introduction DIFFERENTIATE RED EYE DISORDERS • Needs immediate treatment • Needs treatment within a few days • Does not require treatment Introduction SUBJECTIVE EYE COMPLAINTS • Decreased vision • Pain • Redness Characterize the complaint through history and exam. Introduction TYPES OF RED EYE DISORDERS • Mechanical trauma • Chemical trauma • Inflammation/infection Introduction ETIOLOGIES OF RED EYE 1. Chemical injury 2. Angle-closure glaucoma 3. Ocular foreign body 4. Corneal abrasion 5. Uveitis 6. Conjunctivitis 7. Ocular surface disease 8. Subconjunctival hemorrhage Evaluation RED EYE: POSSIBLE CAUSES • Trauma • Chemicals • Infection • Allergy • Systemic conditions Evaluation RED EYE: CAUSE AND EFFECT Symptom Cause Itching Allergy Burning Lid disorders, dry eye Foreign body sensation Foreign body, corneal abrasion Localized lid tenderness Hordeolum, chalazion Evaluation RED EYE: CAUSE AND EFFECT (Continued) Symptom Cause Deep, intense pain Corneal abrasions, scleritis, iritis, acute glaucoma, sinusitis, etc. Photophobia Corneal abrasions, iritis, acute glaucoma Halo vision Corneal edema (acute glaucoma, uveitis) Evaluation Equipment needed to evaluate red eye Evaluation Refer red eye with vision loss to ophthalmologist for evaluation Evaluation RED EYE DISORDERS: AN ANATOMIC APPROACH • Face • Adnexa – Orbital area – Lids – Ocular movements • Globe – Conjunctiva, sclera – Anterior chamber (using slit lamp if possible) – Intraocular pressure Disorders of the Ocular Adnexa Disorders of the Ocular Adnexa Hordeolum Disorders of the Ocular -

A Description of the Clinical Features of Brimonidine- Associated Uveitis Alyssa Louie Primary Care Resident, San Francisco VA
Drug-induced intraocular inflammation: A description of the clinical features of brimonidine- associated uveitis Alyssa Louie Primary Care Resident, San Francisco VA Abstract: A description of the clinical features, diagnostic work-up, and management of acute anterior uveitis caused by brimonidine, a widely used glaucoma medication. I. Case History a. Patient demographics: 74 year-old white male b. Chief complaint: eye pain, redness, irritation for last 2 weeks c. Ocular and medical history: i. Ocular history 1. Primary open angle glaucoma OU, diagnosed 8 years ago 2. Senile cataracts OU, not visually significant 3. Type 2 Diabetes without retinopathy OU 4. No prior history of uveitis ii. Medical history: Diabetes Mellitus Type 2 iii. No known drug allergies d. Medications i. Ocular: dorzolamide BID OU (1.5 years), brimonidine BID OU (11 months), travatan QHS OU (5.5 years) ii. Medical: metformin 500mg tab BID PO II. Pertinent Findings a. Clinical exam i. Visual acuities: OD 20/20-, OS 20/20- ii. Goldmann applanation tonometry: 13 mm Hg OD, 13 mm Hg OS iii. Anterior segment 1. OU: 3+ diffuse conjunctival injection 2. OU: central and inferior granulomatous keratic precipitates 3. OU: Grade 1+ cell, 1+ flare 4. OU: No synechiae or iris changes were present iv. Posterior segment 1. Optic Nerve a. OD: Cup-to-disc ratio 0.70H/V, distinct margins b. OS: Cup-to-disc ratio 0.75H/V, distinct margins 2. Posterior pole, periphery, vitreous: unremarkable OU b. Laboratory Studies i. ACE, Lysozyme, FTA-ABS, VDRL, HLA-B27, Rheumatoid Factor, ANA, PPD, Chest X- ray: all negative/unreactive III. -

Characteristics of Allergy in Autoimmune Thyroid Diseases Ildikó
Characteristics of allergy in autoimmune thyroid diseases Ildikó Molnár MD, PhD, EndoMed, Hungary ImmunSum, Baltimore, 2014 Relationship between allergic responses and thyroid autoimmunity IgE levels IgE deposits are present in Graves’ thyroid and orbital tissues (Werner SC et al., N Engl Med, 1972;287:421-425.; Raikow RB et al., Ophthalmol. 1990; 97:629-635.) Elevated IgE levels associated with hyperthyroid Graves’ disease (Akira S et al., J Clin Endocrinol Metab 1999; 84:3602-3605.; Takashi Y et al., J Clin Endocrinol Metab 2000; 85:2775- 2778.) Evidence of immunglobulin E autoantibodies to thyrotropin receptor (TSH rec) and thyroid peroxidase (TPO) (Metcalfe R et al., J Clin Endocrinol Metab 2002;87:1754-1761.; Gou J et al., Clin Immunol Immunopathol 1997; 82: 157-162.) Th2-derived cytokine profils Elevated serum levels of IL-5 and IL-13 cytokines. (Hidaka Y et al., Thyroid 1998; 8:235-239.; Ichiro K et al., J Clin Endocrinol Metab 2001; 86:3540-3544.) Allergic rhinitis associated frequently with Graves’ disease (Amino N et al., Thyroid 2003; 13:811-814.; Hidaka Y et al., Thyroid 1996; 6: 349-351.) Common key factors regulate the immune responses in both allergic and autoimmune conditions (Rottem M et al., Dev Immunology 2002; 9: 161-167.) ImmunSum, Baltimore, 2014 Previous results Graves’ ophthalmopathy associated with increased total IgE serum levels. Molnár I et al., Eur J Med Rev 1996; 1:543-546. Hyperthyroid Graves’ ophthalmopathy demonstrated elevated serum IL-5 levels compared to patients who had no eye signs. Molnár I , Abstract: ACT International Suppl., 2000; 2: 220. Decreased serum levels of nerve growth factor (NGF) associated with hyperthyroid Graves’ ophthalmopathy compared to those who had no eye signs. -

Pediatric Pharmacology and Pathology
7/31/2017 In the next 2 hours……. Pediatric Pharmacology and Pathology . Ocular Medications and Children The content of th is COPE Accredited CE activity was prepared independently by Valerie M. Kattouf O.D. without input from members of the optometric community . Brief review of examination techniques/modifications for children The content and format of this course is presented without commercial bias and does not claim superiority of any commercial product or service . Common Presentations of Pediatric Pathology Valerie M. Kattouf O.D., F.A.A.O. Illinois College of Optometry Chief, Pediatric Binocular Vision Service Associate Professor Ocular Medications & Children Ocular Medications & Children . Pediatric systems differ in: . The rules: – drug excretion – birth 2 years old = 1/2 dose kidney is the main site of drug excretion – 2-3 years old = 2/3 dose diminished 2° renal immaturity – > 3 years old = adult dose – biotransformation liver is organ for drug metabolism Impaired 2° enzyme immaturity . If only 50 % is absorbed may be 10x maximum dosage Punctal Occlusion for 3-4 minutes ↓ systemic absorption by 40% Ocular Medications & Children Ocular Medications & Children . Systemic absorption occurs through….. Ocular Meds with strongest potential for pediatric SE : – Mucous membrane of Nasolacrimal Duct 80% of each gtt passing through NLD system is available for rapid systemic absorption by the nasal mucosa – 10 % Phenylephrine – Conjunctiva – Oropharynx – 2 % Epinephrine – Digestive system (if swallowed) Modified by variation in Gastric pH, delayed gastric emptying & intestinal mobility – 1 % Atropine – Skin (2° overflow from conjunctival sac) Greatest in infants – 2 % Cyclopentalate Blood volume of neonate 1/20 adult Therefore absorbed meds are more concentrated at this age – 1 % Prednisone 1 7/31/2017 Ocular Medications & Children Ocular Medications & Children . -
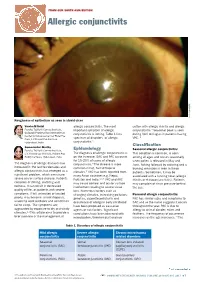
Allergic Conjunctivits VPEI Rathi/ L V (C)
FROM OUR SOUTH ASIA EDITION Allergic conjunctivits VPEI L Rathi/ V (c) Roughness of epithelium as seen in shield ulcer Varsha M Rathi allergic conjunctivitis. The most cotton with allergic rhinitis and allergic Faculty, Tej Kohli Cornea Institute, important symptom of allergic conjunctivitis.8 Seasonal peak is seen Gullapalli Pratibha Rao International conjunctivitis is itching. Table 1 lists during April to August in patients having Center for Advancement of Rural Eye 9 Care, L V Prasad Eye Institute, spectrum of disorders of allergic VKC. 3 Hyderabad, India conjunctivitis. Classification Somasheila I Murthy Faculty, Tej Kohli Cornea Institute, Epidemiology Seasonal allergic conjunctivitis: L.V. Prasad Eye Institute, Kallam Anji The diagnosis of allergic conjunctivitis is This condition is common, is seen Reddy Campus, Hyderabad, India on the increase. SAC and PAC accounts among all ages and occurs seasonally for 15-20% of cases of allergic when pollen is released in May and The diagnosis of allergic diseases has conjunctivitis.4 The disease is more June. Itching followed by watering and a increased in the last few decades and common in hot, humid tropical burning sensation is seen in these allergic conjunctivitis has emerged as a climates.5 VKC has been reported from patients. Sometimes, it may be significant problem, which can cause many Asian countries e.g. Nepal, associated with a running nose (allergic severe ocular surface disease. Patients Pakistan and India.2,6,7 VKC and AKC rhinitis or rhinoconjunctivitis). Patients complain of itching, watering and may cause corneal and ocular surface may complain of sinus pressure behind redness. It can result in decreased involvement leading to severe visual the eye. -

Acquired Etiologies of Lacrimal System Obstructions
5 Acquired Etiologies of Lacrimal System Obstructions Daniel P. Schaefer Acquired obstructions of the lacrimal excretory outfl ow system will produce the symptoms of epiphora, mucopurulent discharge, pain, dacryocystitis, and even cellulitis, prompting the patient to seek the ophthalmologist for evaluation and treatment. Impaired tear outfl ow may be functional, structural, or both. The causes may be primary – those resulting from infl ammation of unknown causes that lead to occlusive fi brosis—or secondary, resulting from infections, infl amma- tion, trauma, malignancies, toxicity, or mechanical causes. Secondary acquired dacryostenosis and obstruction may result from many causes, both common and obscure. Occasionally, the precise pathogenesis of nasolacrimal duct obstruction will, despite years of investigations, be elusive. To properly evaluate and appropriately treat the patient, the ophthal- mologist must have knowledge and comprehension of the lacrimal anatomy, the lacrimal apparatus, pathophysiology, ocular and nasal relationships, ophthalmic and systemic disease process, as well as the topical and systemic medications that can affect the nasolacrimal duct system. One must be able to assess if the cause is secondary to outfl ow anomalies, hypersecretion or refl ex secretion, pseudoepiphora, eyelid malposition abnormalities, trichiasis, foreign bodies and conjunctival concretions, keratitis, tear fi lm defi ciencies or instability, dry eye syn- dromes, ocular surface abnormalities, irritation or tumors affecting the trigeminal nerve, allergy, medications, or environmental factors. Abnormalities of the lacrimal pump function can result from involu- tional changes, eyelid laxity, facial nerve paralysis, or fl oppy eyelid syndrome, all of which displace the punctum from the lacrimal lake. If the cause is secondary to obstruction of the nasolacrimal duct system, the ophthalmologist must be able to determine where the anomaly is and what the cause is, in order to provide the best treatment possible for the patient. -

Review Article Conjunctivitis
Review Article Conjunctivitis: A Systematic Review Amir A. Azari, MD1,2, Amir Arabi, MD, MPH1,2 1Ophthalmic Research Center, Research Institute for Ophthalmology and Vision Science, Shahid Beheshti University of Medical Sciences, Tehran, Iran 2Department of Ophthalmology, Torfeh Medical Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran ORCID: Amir A. Azari: https://orcid.org/0000-0003-2013-3284 Abstract Conjunctivitis is a commonly encountered condition in ophthalmology clinics throughout the world. In the management of suspected cases of conjunctivitis, alarming signs for more serious intraocular conditions, such as severe pain, decreased vision, and painful pupillary reaction, must be considered. Additionally, a thorough medical and ophthalmic history should be obtained and a thorough physical examination should be done in patients with atypical findings and chronic course. Concurrent physical exam findings with relevant history may reveal the presence of a systemic condition with involvement of the conjunctiva. Viral conjunctivitis remains to be the most common overall cause of conjunctivitis. Bacterial conjunctivitis is encountered less frequently and it is the second most common cause of infectious conjunctivitis. Allergic conjunctivitis is encountered in nearly half of the population and the findings include itching, mucoid discharge, chemosis, and eyelid edema. Long-term usage of eye drops with preservatives in a patient with conjunctival irritation and discharge points to the toxic conjunctivitis as the underlying etiology. Effective management of conjunctivitis includes timely diagnosis, appropriate differentiation of the various etiologies, and appropriate treatment. Keywords: Allergic; Bacterial; Conjunctivitis; COVID-19; Coronavirus; Viral; Toxic J Ophthalmic Vis Res 2020; 15 (3): 372–395 INTRODUCTION by engorgement of the blood vessels, ocular discharge, and pain. -
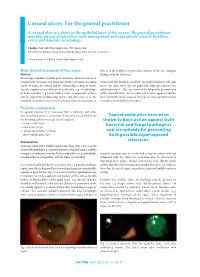
Corneal Ulcers: for the General Practitioner
Corneal ulcers: For the general practitioner A corneal ulcer is a defect in the epithelial layer of the cornea. e general practitioner may play an important role in early management and appropriate referral. Incidence varies and depends on aetiology. S Ballim, MB ChB, Dip Ophth (SA), FC Ophth (SA) Department of Ophthalmology, University of KwaZulu-Natal, Durban, South Africa Correspondence to: S Ballim ([email protected]) Basic clinical assessment of the cornea clue as to the health of the posterior segment of the eye. Compare History ndings with the fellow eye. Presenting complaints include pain, irritation, decreased vision or foreign-body sensation. Ask about any history of trauma, including Fluorescein dye should be instilled. Any epithelial defects will stain nature of injury, the timing and the relationship to drop in vision. green. e stain shows up best under blue light (present on some Specic enquiry as to instillation of medication (e.g. steroid drops) ophthalmoscopes). is can, however, be adequately demonstrated or home remedies (e.g. breast milk or urine) is important, as these with a standard torch. e tear lm will become apparent and the may be important predisposing factors and give clues as to the lacus lacrimalis can be assessed. Dry eye is a very signicant factor aetiology. Contact lens use is a very common cause of corneal ulcers. in many corneal epithelial disorders. Tools for examination In general practice it is presumed that a slit-lamp and other specialised equipment is unavailable. It would be reasonable to have Topical antibiotics have been the following ophthalmic tools at one’s disposal: shown to have action against both • visual acuity chart bacterial and fungal pathogens • uorescein strips • topical anaesthetic eye drops and are suitable for preventing • direct ophthalmoscope. -

Atopic Keratoconjunctivitis
Atopic Keratoconjunctivitis Fahd Anzaar, MD, C. Stephen Foster, MD, FACS, FACR A 31 year old male patient presented to us, referred from an ophthalmologist in New York, with a diagnosis of ‘recent corneal tear in the right eye’. The patient complained of persistent ‘soreness’, excessive tearing, and photophobia. He had had these complaints intermittently, alongwith yellowish mucous discharge (especially upon awakening) for many years and many eye drops and oral medications had been prescribed by many ophthalmologists and dermatologists over the years. This particular episode was severe and unremitting, and hence the referral. The patient’s past medical history was significant for atopic dermatitis (AD or eczema) and exercise-induced asthma. The family history was significant for myocardial infarction in his father, and cancer, specifically colorectal cancer, and the patient’s mother had ovarian cancer. The patient was a non-smoker, was a student working part time as a waiter, and occasionally drank alcohol. At presentation, his medications included systemic prednisone (60 mg daily), topically applied (skin) tacrolimus cream and triamcinolone. He was also using Lotemax, Alrex and Vigamox eye drops in the right eye four times daily, Restasis in the left eye twice daily, and Celluvisc in both eyes as needed. He was allergic to Dovonex cream, and had a history of intolerance to systemic cyclosporine (hypertension and nephropathy). General physical examination disclosed eczema of the face and chest. Visual acuities were 20/200 OD and hand motion OS, intraocular pressures were 12 and 15, and slit lamp biomicroscopy showed 3+ conjunctival injection in the right eye, with ciliary flushing, and a corneal epithelial defect with stromal opacification, glue, and bilateral keratoconus. -

The Itchy Eye: Diagnosis and Management of Ocular Pruritus
Ophthalmic Pearls EXTERNAL DISEASE The Itchy Eye: Diagnosis and Management of Ocular Pruritus by jocelyn kuryan, md, prabjot channa, md, and roy s. chuck, md, phd edited by ingrid u. scott, md, mph, and sharon fekrat, md cular pruritus is a com- conjunctivitis, giant papillary conjunc- mon symptom that brings tivitis and contact dermatoblepharitis. patients to the ophthalmol- A complete history, review of systems ogist’s office. It may be and examination can help differentiate tempting to overlook this among these etiologies. Oseemingly minor problem while as- sessing patients for potentially vision- Pathophysiology threatening diseases. However, itchy Many of the causes of ocular pruritus eyes can be a major problem and are immunologically mediated. source of anxiety for patients, often af- • Allergic conjunctivitis and AKC fecting their day-to-day quality of life. are a result of IgE-mediated reactions Although a physician’s inclination that cause mast-cell degranulation CONTACT LENS COMPLICATION. Giant may be simply to treat the symptom and histamine release. IgE-mediated papillary conjunctivitis. with topical mast-cell stabilizers or an- processes as well as impaired cellular tihistamines, it is important to identify immunity and genetic factors also con- the action of local inflammatory me- the underlying cause. By carefully and tribute to atopic dermatitis. Both type diators in the setting of hyposecretion methodically determining the etiology I (immediate) and type IV (delayed) and obstruction of the meibomian of ocular pruritus, the clinician can se- hypersensitivity reactions are involved gland orifices. lect an appropriate treatment regimen in vernal keratoconjunctivitis. Con- • DES can be caused by either aque- and provide patients with the relief junctival scrapings from patients with ous tear deficiency (either Sjögren or they seek. -

Case Report of Restoration of the Corneal Epithelium in a Patient With
CORE Metadata, citation and similar papers at core.ac.uk Provided by Elsevier - Publisher Connector Allergology International. 2010;59:309-312 ! DOI: 10.2332 allergolint.10-CR-0185 CASE REPORT Case Report of Restoration of the Corneal Epithelium in a Patient with Atopic Keratoconjunctivitis Resulting in Amelioration of Ocular Allergic Inflammation Ken Fukuda1,2, Naoyuki Yamada1 and Teruo Nishida1 ABSTRACT Background: Atopic and vernal keratoconjunctivitis are severe types of ocular allergic disease characterized not only by conjunctival inflammation but also by corneal involvement. In vitro studies have suggested that breakdown of corneal epithelial barrier function and subsequent activation of stromal fibroblasts may amplify ocular allergic inflammation. Case Summary: A 27-year-old man with atopic dermatitis developed atopic keratoconjunctivitis including cor- neal ulcer with plaque deposition in his right eye. Conjunctival inflammation in the right eye was resistant to topical steroid therapy. Surgical removal of corneal plaque and administration of autologous fibronectin eye- drops resulted not only in resurfacing of the corneal epithelium but also in amelioration of conjunctival inflam- mation. Discussion: This case suggests that loss of corneal epithelial integrity likely exacerbates conjunctival allergic inflammation and that restoration or maintenance of the barrier function of the corneal epithelium may be one of the important targets for the treatment of severe ocular allergic diseases. KEY WORDS allergic inflammation, atopic keratoconjunctivitis, conjunctiva, corneal ulcer, epithelium The pathogenesis of corneal lesions is thought to INTRODUCTION involve a combination of toxic epitheliopathy due to Atopic keratoconjunctivitis (AKC) and vernal kerato- eosinophil- and mast cell-derived inflammatory me- conjunctivitis (VKC) are severe types of ocular aller- diators as well as mechanical damage to the corneal gic disease that are characterized by chronic inflam- epithelium by giant papillae. -

Allergic Conjunctivitis
Allergic Conjunctivitis Allergic Conjunctivitis MC Sánchez1, B Fernández Parra2, V Matheu3, A Navarro4, MD Ibáñez5, I Dávila6, MT Dordal7, M Lluch Bernal8, C Rondón9, J Montoro10, E Antón11, C Colás12, A Valero13,14 (SEAIC Rhinoconjuntivitis Committee 2010) 1Allergology Unit, C.E. Virgen de la Cinta, Hospital Juan Ramón Jiménez, Huelva, Spain 2Allergology Unit, Hospital El Bierzo, Ponferrada, León, Spain 3Department of Allergology, Hospital Universitario Nuestra Señora de Candelaria, Tenerife, Spain 4Allergology Unit, Hospital El Tomillar, UGC Intercentros de Alergología Valme-Rocío, Seville, Spain 5Department of Allergology, Hospital Infantil Universitario Niño Jesús, Madrid, Spain 6Department of Allergology, Hospital Universitario, Salamanca, Spain 7Department of Allergology, Fundación Sanitaria Sant Pere Claver, Barcelona, Spain 8Department of Allergology, Complejo Hospitalario de Toledo, Toledo, Spain 9Department of Allergology, Hospital Carlos Haya, Málaga, Spain 10Allergy Unit, Hospital Universitario Arnau de Vilanova, Valencia 11Department of Allergology, Hospital Universitario Marqués de Valdecilla, Santander, Spain 12Department of Allergology, Hospital Clínico Universitario, Zaragoza, Spain 13Allergy Unit, Department of Pneumology, Hospital Clínic i Universitari. Barcelona, Spain 14Centro de Investigación Biomédica en Red (CIBER) de Enfermedades Respiratorias, Barcelona, Spain ■ Abstract Interest in allergic conjunctivitis (AC), isolated or associated to allergic rhinitis, has increased in recent years due to its high and growing prevalence, the important healthcare costs generated by the disease, and its impact upon patient quality of life. A review is made of the immunopathological mechanisms of AC, its diagnosis and the differential diagnosis with other ophthalmological allergic disorders. The current management of AC is based on minimizing contact of the causal allergen with the conjunctiva using a series of protective measures, and on controlling the symptoms produced by the allergic infl ammatory process.