Biodiversity of Statistical Correlation Between Fungal Population And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Government of India Ministry of Housing & Urban Affairs
GOVERNMENT OF INDIA MINISTRY OF HOUSING & URBAN AFFAIRS LOK SABHA UNSTARRED QUESTION No. 2503 TO BE ANSWERED ON JANUARY 2, 2018 URBAN INFRASTRUCTURE PROJECTS No. 2503. SHRI R. GOPALAKRISHNAN: Will the Minister of HOUSING & URBAN AFFAIRS be pleased to state: (a) whether the Government has granted approval and released funds for implementing a number of urban infrastructure projects of Tamil Nadu; (b) if so, the details thereof along with the funds allocated/released for the said purpose during the last three years and the current year, city-wise including Madurai city in Tamil Nadu; and (c) the present status of those projects and the steps taken/being taken for expediting these projects? ANSWER THE MINISTER OF STATE (INDEPENDENT CHARGE) IN THE MINISTRY OF HOUSING & URBAN AFFAIRS (SHRI HARDEEP SINGH PURI) (a) to (c) Yes Madam. The Ministry of Housing & Urban Affairs has approved and released funds for implementing urban infrastructure projects in Tamil Nadu under its various schemes, viz., Atal Mission for Rejuvenation and Urban Transformation (AMRUT), Smart Cities Mission (SCM), Page 1 of 2 Heritage City Development and Augmentation Yojana (HRIDAY), Swacchh Bharat Mission – Urban [SBM (U)], Urban Infrastructure Development in Satellite Towns around Seven Mega Cities (UIDSST), Urban Transport (UT), Pradhan Mantri Awas Yojana-Urban [PMAY (U)] and Jawaharlal Nehru National Urban Renewal Mission (JnNURM). Under AMRUT, the Ministry of Housing & Urban Affairs does not approve projects for individual cities but accords approval to the State Annual Action Plans (SAAPs) only. Selection, approval and implementation of individual projects is done by State Government. Further, the Ministry of Housing & Urban Affairs does not release central share of funds city-wise, but funds are released State-wise. -
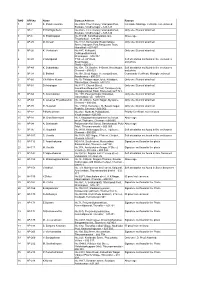
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Tomato Prices to Be Stable for Next One Month
Tamil Nadu Agricultural University Coimbatore – 641 003 Dr. E. Somasundaram, Ph.D., Phone: 0422 - 6611302 Public Relations Officer & Fax: 0422 – 2431821 Professor (Agronomy) E-mail: [email protected] To Date: 18-11-2013 The Editor, Sir, I request that the following matter may kindly be published in your esteemed daily: Tomato prices to be stable for next one month Tomato ranks second after potato in world consumption. The major tomato growing countries are China, USA, Italy, Turkey, India and Egypt. At the world level, 159.34 million Tonnes of tomatoes are produced in an area of 4.75 million ha and the average productivity of the crop is 33.53 t/ha. China stands first with a contribution of 32 percent to world tomato production. India stands second with 9.47 percent production globally. There is a sizeable increase in acreage and production of tomato in India during the past five years. Area increased from 5.96 lakh ha in 2006-07 to 8.65lakh ha.in 2010-11, while the production increased from 10.05 to 16.8 million tonnes with a productivity increase from 16.9 to 19.5 tons/ha. Tomato has a very good demand in export markets too. Pakistan is the major consumer for Indian tomatoes followed by United Arab Emirates, Bangladesh and Nepal. In India, Andhra Pradesh has the highest production share with 35 percent followed by Karnataka with 10.44 percent. Tamil Nadu stands ninth place with 3.45 percent of total tomato production. In terms of productivity Karnataka occupies first place with 34.3 t/ha. -

2019060452.Pdf
DISTRICT SURVEY REPORT FOR GRANITE INDEX Page Chapter Content No. 1. Introduction 4 2. Overview of Mining Activity in the District 7 3. General Profile of the District 8-9 4. Geology of Dharmapuri District 10-24 5. Drainage of Irrigation pattern 25-26 6. Land Utilisation Pattern in the District: Forest, Agricultural, 26-30 Horticultural, Mining etc., 7. Surface Water and Ground Water Scenario of the District 31-34 8. Climate and Rainfall of the District 34-36 9. Details of Mining Leases in the District 37-42 10. Details of Royalty or Revenue Received in last three years 43 11. Details of Production of Minor Mineral in last three years 44 12. Mineral Map of the District 45 13. List of Letter of Intent (LOI) Holder in the District along with 46 its validity 14. Total Mineral Reserve Available in the District 47 15. Quality/Grade of Mineral available in the District 47-48 16. Use of Mineral 48 17. Demand and Supply of the Mineral in the last three years 48 18. Mining Leases Marked on the Map of the District 49 19. Details of the area of where there is a Cluster of the Mining 50 Leases 20. Details of Eco-Sensitive Area 50-51 21. Impact on the Environment Due to Mining activity 51-53 22. Remedial measures to Mitigate the Impact of Mining on the 54-55 Environment 23. Reclamation of the Mined Out Area 56 24. Risk assessment & Disaster Management Plan 57-59 25. Details of Occupational Health Issue in the District 60 26. -
DISTRICT SURVEY REPORT for ROUGH STONE INDEX Page Chapter Content No
DISTRICT SURVEY REPORT FOR ROUGH STONE INDEX Page Chapter Content No. 1. Introduction 4 2. Overview of Mining Activity in the District 7 3. General Profile of the District 8-9 4. Geology of Dharmapuri District 10-21 5. Drainage of Irrigation pattern 22-23 6. Land Utilisation Pattern in the District: Forest, Agricultural, 23-27 Horticultural, Mining etc., 7. Surface Water and Ground Water Scenario of the District 28-31 8. Climate and Rainfall of the District 32-34 9. Details of Mining Leases in the District 35-41 10. Details of Royalty or Revenue Received in last three years 42 11. Details of Production of Minor Mineral in last three years 43 12. Mineral Map of the District 44 13. List of Letter of Intent (LOI) Holder in the District along with 45 its validity 14. Total Mineral Reserve Available in the District 46 15. Quality/Grade of Mineral available in the District 46 16. Use of Mineral 47 17. Demand and Supply of the Mineral in the last three years 47 18. Mining Leases Marked on the Map of the District 48 19. Details of the area of where there is a Cluster of the Mining 49 Leases 20. Details of Eco-Sensitive Area 49-50 21. Impact on the Environment Due to Mining activity 50-52 22. Remedial measures to Mitigate the Impact of Mining on the 53-54 Environment 23. Reclamation of the Mined Out Area 55 24. Risk assessment & Disaster Management Plan 56-58 25. Details of Occupational Health Issue in the District 59 26. -

Pre-Feasibility Report of Black Granite (Dolerite) Quarry
Pre-Feasibility Report of Black Granite (Dolerite) quarry (Under the Guidelines of Ministry of Environment and Forest in terms of the provisions of EIA notification 20051, 52 & 127 and specifically in circular No J-11013/41/20051, 52 & 127 -IA.II (I) dated 30th December, 2010) Location of the Quarry S.F.No. 292/2D2, 294/1(P), 115/2B2 (P) & 115/2C (P) Samanur & Gummanur Village, Palacode Taluk, Dharmapuri District, Extent: 1.58.3 Ha (less than 5Ha) Category: B2 Project Applicant Thiru.P.Raman, S/o.Periyasamy Gounder, 5/11B, Theerthagiri Nagar Palacode Town&Taluk, Dharmapuri District. Pre feasibility report of Samanur & Gummanur Black Granite quarry Thiru.P.Raman Under EIA Notification 2006 EXECUTIVE SUMMARY: This present Mining Plan is prepared in respect of Black Granite (Dolerite) quarry belongs to Thiru.P.Raman. The Precise area Communication has been granted as per Govt. letter No. 10852/MME.2/2017-1,Dated:21.09.2017 for over an Extent of 1.58.3 Ha located in S.F.Nos. 292/2D2, 294/1(P), 115/2B2 (P) & 115/2C (P) of Samanur & Gummanur Village, Palacode Taluk and Dharmapuri District for during this period subjected to submission of Environmental clearance from SEIAA / DEIAA, consent for Establishment and Consent for operation from TNPCB. SALIENT FEATURES OF THE PROJECT S.NO PARTICULAR DETAILS 1 Name of the Proponent Thiru.P.Raman 2 Type of Project Black Granite (Dolerite) Survey No. 292/2D2, 294/1(P), 115/2B2 (P) & 115/2C (P) Samanur & Gummanur village, 3 Location Palacode Taluk, Dharmapuri district, Tamilnadu state. -

Nmipe Ii,1Ui
ii,1ui 3TrrTrr 31t T1 rr , , nMIPE YVAHE Oc CULIHEATHH * * THE MAHATWA Building Materials & Technology Promotion Council Ministry of H.sing & Urban Affairs Government of tndi Ref: BMT/C-F/EBR-PMAY/201 7-18/87 25 March, 2019 To The Principal Secretary Tamil Nadu Slum Clearance Board Government of Tamil Nadu 5, Kamarajar Salal, Chepauk, Chennai-600005 Subject: Release of Central Assistance under Housing for All (Urban) Pradhan Mantri Awas Yojna from National Urban Housing Fund (NUHF) - reg. Sir, Please refer to the sanction letter no. N-1101217412018-HFA-IIl-UD (EN 9052999)dated 25.03.2019 on the subject mentioned above received from Ministry of Housing & Urban Affairs (copy enclosed). In this regard, it is informed that an amount of Rs. 92,97,60,000/- (Rupees Ninety Two Crore-Ninety Seven Lakh and Sixty Thousand Only)has been credited to the State Government of Tamil Nadu, as mentioned in the sanction order as Central Assistance under Housing for All (Urban)Pradhan Mantri Awas Yojna. The amount was released through EAT module of PFMS in the account mentioned in the above order. Thanking you, Yours faithfully, (Dr. Shailes Kr. Agrawal) Executive Director End.: As above Copy to: I. The Chief Controller of Accounts, Ministry of Housing & Urban Affairs, Nirman Bhawan, New Delhi- I 10011 -2T Sh. S. C. Jana, Dy. Secretary (HFA-111), Mission Director, Ministry of Housing & Urban Affairs, Nirman Bhawan, New Delhi-110011 3. The Under Secretary, HFA -Ill Section, Ministry of Housing & Urban Affairs, Nirman Bha wan New Delhi CD ç o F1PF ThJ iTPT 5'-T -i ., Core 5A, 1 Floor, India Habitat Centre, oaJ lNe w DeUh i 110 03. -

Dharmapuri District Statistical Hand Book (2007-08)
DHARMAPURI DISTRICT STATISTICAL HAND BOOK (2007-08) 1. AREA AND POPULATION 2. CLIMATE AND RAINFALL 3. AGRICULTURE 4. IRRIGATION 5. ANIMAL HUSBANDARY 6. BANKING AND INSURANCE 7. CO-OPERATION 8. CIVIL SUPPLIES 9. COMMUNICATION 10. ELECTRICITY 11. EDUCATION 12. FISHERIES 13. HANDLOOM 14. HANDICRAFTS 15. HOPITAL 16. HOUSING 17. INDUSTRIES 18.FACTORIES 19. LOCAL BODIES 20. LABOUR AND EMPLOYMENT 21. LEGAL SERVICES 22. LIBRARIES 23. MINING AND QUARYING 24. MANUFACTURING SECTOR 27. NON-CONVENTIONAL ENERGY 25. MEDICAL SERVICES 26. MOTOR VEHICLES GENERATION 28. POLICE AND PRISONS 29. PUBLIC HEALTH 30. PRINTING AND PUBLICATION 31.PRICES – INDICES 32. QUALITY CONTROL 33. REGISTRATION 36. RECREATION AND CULTURAL 35. RESTAURANTS AND HOTELS 34.REPAIRS AND SERVICE SERVICES 39. SCIENTIFIC AND RESEARCH 37. SOCIAL WELFARE 38.SANITARY SERVICES SERVICE 40. STORAGE FACILITIES 41.TEXTILES 42.TRADE AND COMMERCE 43.TRANSPORT 44. TOURISM 45.VITAL STATISTICS 46. VOLUNTARY SERVICES 47. WATER WORK AND SUPPLY 8. 7. 6. 5. 4. 3. 2. 1. (1 No Sl. 1.1 Morapur Total Total Pappireddipatty Pappireddipatty Karimangalam Harur Nallampalli Dharmapuri Dharmapuri Pennagaram Palacode Palacode Name of the MUNICIPALITIES AREA, POPULATION, LITERATES,SC’S,ST’S–SEXWIS Blocks (2) 4498 348 384 1130 408 562 882 413 371 (3) Area (sq.km) 1295182 137506 164074 194882 102866 155000 165736 161343 213775 (4) Persons Population 1.AREA ANDPOPULATION (5 670520 71131 84923 102269 52646 80073 85174 84202 110102 Male Source: of Census 2001 India 624662 66375 79151 92613 50220 74927 80562 77141 103673 -
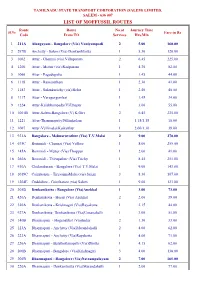
LIST of MOFFUSSIL ROUTES Route Route No.Of Journey Time Sl.No Fare in Rs Code from to Services Hrs.Min
TAMILNADU STATE TRANSPORT CORPORATION (SALEM) LIMITED, SALEM - 636 007 LIST OF MOFFUSSIL ROUTES Route Route No.of Journey Time Sl.No Fare in Rs Code From TO Services Hrs.Min 1 211A Alangayam - Bangalore (Via) Vaniyampadi 2 5.00 160.00 2 297B Anchetty - Salem (Via) Denkanikkotta 1 5.30 120.00 3 1062 Attur - Chennai (via) Villupuram 2 6.45 225.00 4 1200 Attur - Mettur (via) Rasipuram 1 4.30 82.00 5 1066 Attur - Pagudupattu 1 1.45 44.00 6 1118 Attur - Ramanatham 1 2.30 41.00 7 1183 Attur - Sulankurichy (via) Belur 1 2.40 40.00 8 1117 Attur - Varagurgombai 1 1.45 34.00 9 1224 Attur-Kalakkampady(V)Ethapur 1 3.00 55.00 10 1001B Attur-Salem-Bangalore (V) K.Giri 2 6.45 235.00 11 1221 Attur-Thammapatty/Pillankulam 1 1.15/1.15 18.00 12 1067 Attur-Vellimalai/Kaikalthur 1 2.00/1.30 38.00 13 921A Bangalore - Melmaruvathur (Via) T.V.Malai 2 9.00 270.00 14 675C Bommidi - Chennai (Via) Vellore 1 8.00 255.00 15 145A Bommidi - Mettur (Via) Thoppur 1 2.00 41.00 16 260A Bommidi - Thirupathur (Via) Trichy 1 8.45 251.00 17 930A Chidambaram - Bangalore (Via) T.V.Malai 1 9.00 143.00 18 1019C Coimbatore - TiruvannaMalai (via) Salem 3 8.30 107.00 19 1204E Cuddalore - Coimbatore (via) Salem 1 9.00 123.00 20 305B Denkanikotta - Bangalore (Via)Anekkal 1 3.00 73.00 21 420A Denkanikotta - Hosur (Via) Anekkal 2 2.00 39.00 22 320A Denkanikotta - Krishnagiri (Via)Rayakotta 1 2.15 44.00 23 927A Denkanikotta- Denkanikotta (Via)Unsanahalli 1 3.00 51.00 24 140B Dharmapuri - Hogenakkal (Via)Indur 2 1.30 33.00 25 123A Dharmapuri - Anchetty (Via)Marandahalli 2 4.00 62.00 26 -

Technology Mapping and Adoption Behaviour for Sugarcane Protection Technologies by Dharmapuri District Sugarcane Growers
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Journal of Applied and Natural Science Journal of Applied and Natural Science 11(4): 806-809 (2019) ISSN : 0974-9411 (Print), 2231-5209 (Online) journals.ansfoundation.org Technology mapping and adoption behaviour for sugarcane protection technologies by Dharmapuri District Sugarcane growers C. Karpagam* Central Institute for Cotton Research, Regional Station, Coimbatore – 641003 (Tamil Article Info Nadu), India https://doi.org/ M. K. Selvam 10.31018/jans.v11i4.2186 Sugarcane Breeding Institute, Coimbatore - 641041 (Tamil Nadu), India Received: October 31, 2019 P. Mooventhan Revised: December 2, 2019 Accepted: December 5, 2019 National Institute of Biotic Stress Management, Raipur - 493225 (Chhattisgarh), India *Corresponding author. E-mail: [email protected] How to Cite Abstract Karpagam, C. et al. (2019). Sugarcane is the second most important industrial crop in the country occupying about 5 Technology mapping and million hectares of area with the production of 376.9 mt. Although more than 40% of the adoption behaviour for cane area in the country is in Uttar Pradesh, Tamil Nadu ranks first in productivity of sugarcane protection tech- sugarcane. Even though Tamil Nadu is in higher productivity zone, the average farm nologies by Dharmapuri level potential yield was very less which leads lot of scope for increasing production District Sugarcane grow- ers. Journal of Applied and in Tamil Nadu. Sugarcane farmers from Tamil Nadu ranged from small to large. All Natural Science, 11(4): the farmers not following all the recommended practices. Hence, a study is required 806 - 809 https://doi.org/ to analyses the predominant technologies in the particular area and adoption behav- ior of the farmers to bridge the technological gap. -

6.4 Railways 6.4.1Sector Overview
6.4 Railways 6.4.1 Sector Overview The CBIC Area has a dense railway network comprising 2,806 route km, all of which is broad gauge (1,676 mm) – please see a network illustration in Figure 6.4.1. The network is maintained and operated by three zonal railway organizations under the Ministry of Railways (MOR). The largest component of the network is that of the South Western Railway (1,285 route km), operated from a base in Hubli, Karnataka. The Southern Railway, based in Chennai, operates 950 route km and the South Central Railway, with a headquarters in Secunderabad, operates 566 route km within the CBIC boundary. Passengers dominate the traffic on this network, particularly on the Southern Railway portion which generates most of its revenue from passenger traffic. The network consists of a central east-west route of 675 km linking Chennai with Chitradurga, via Bengaluru, plus another 21 routes generally running north and south from Chennai or Bengaluru. The network has the following functions: It connects inland industrial centres with east coast ports in Chennai, Ennore, Krishnapatnam, and, in the future, with new ports in Kattupalli and (possibly) Duggirajapatnam It connects the CBIC area to the major cities of India It connects the inland industrial centres of CBIC with other manufacturing centres located outside the CBIC Area. In particular, the CBIC railway network provides some important trunk line connections between: Chennai and Gudur Junction (a “Golden Quadrilateral” connecting Chennai with Delhi and Kolkata); Chennai, Renigunta and Nandalur (also a “Golden Quadrilateral” connecting Chennai with Hyderabad and Mumbai); Chennai-Salem, Chennai-Villupuram and Bengaluru-Mysore (links with major cities and with agricultural areas in the south); Bengaluru and Dharmavaram (links to Hyderabad, Mumbai and Delhi, as well as the steel producing region around Bellary); and Bengaluru and Chitradurga/Rayadurga (links to Mangalore Port to the southwest and Bellary to the north). -

4D-Modeling of Indian Continental Evolution Through a Geophysical Window: Geological Constraints
Mahadevan, T.M. 2003. 4D-modeling Of Indian Continental Evolution Through A Geophysical Window: Geological Constraints. Journal of the Virtual Explorer 12, 1-29. 4D-modeling Of Indian Continental Evolution Through A Geophysical Window: Geological Constraints. T.M. MAHADEVAN1 1Sree Bagh, Ammankoil Road, Kochi- 682 035. [email protected] Abstract: The paper discusses the contrasting trends in the Precambrian and Phanerozoic evolution of two regions in the southern (region I) and the central parts of the Indian shield (Region II) selected on the basis of the relatively richer geological and geophysical database available. Both regions span two or three orogenies during the whole of the Late Archaean and the Proterozoic and region II has witnessed major thermal episodes of the Late Mesozoic. Current models of tectonics based on empirical considerations of converging reflections or a bi-modal gravity feature, not specifically related to time but based on broad concepts of principles of uniformitarianism, are simplistic and ignore the multi-genetic possibilities of geophysical signatures in a poly- orogenic setting. Region I had a dichronous evolution, wherein the exhumed high-grade domain was a distinct open system that intimately interacted with the magmatic and thermal evolution of the mantle through the whole of the Precambrian. The region II, however, lacks the polarity of a distinctive Proterozoic high-grade domain and exposes dominantly low to mid-crustal greenschist- amphibolite facies regimes with bands of exhumed granulite facies rocks. Shallower crustal segments preserve near surface distensional basinal structures (such as the Vindhyan and Gondwana basins) overprinted penetratively by Phanerozoic Rajamahal and Deccan basic – alkaline magmatism.