Research and Development at Shionogi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -

Factset-Top Ten-0521.Xlsm
Pax International Sustainable Economy Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight ASML Holding NV 34,391,879.94 4.3 Roche Holding Ltd 28,162,840.25 3.5 Novo Nordisk A/S Class B 17,719,993.74 2.2 SAP SE 17,154,858.23 2.1 AstraZeneca PLC 15,759,939.73 2.0 Unilever PLC 13,234,315.16 1.7 Commonwealth Bank of Australia 13,046,820.57 1.6 L'Oreal SA 10,415,009.32 1.3 Schneider Electric SE 10,269,506.68 1.3 GlaxoSmithKline plc 9,942,271.59 1.2 Allianz SE 9,890,811.85 1.2 Hong Kong Exchanges & Clearing Ltd. 9,477,680.83 1.2 Lonza Group AG 9,369,993.95 1.2 RELX PLC 9,269,729.12 1.2 BNP Paribas SA Class A 8,824,299.39 1.1 Takeda Pharmaceutical Co. Ltd. 8,557,780.88 1.1 Air Liquide SA 8,445,618.28 1.1 KDDI Corporation 7,560,223.63 0.9 Recruit Holdings Co., Ltd. 7,424,282.72 0.9 HOYA CORPORATION 7,295,471.27 0.9 ABB Ltd. 7,293,350.84 0.9 BASF SE 7,257,816.71 0.9 Tokyo Electron Ltd. 7,049,583.59 0.9 Munich Reinsurance Company 7,019,776.96 0.9 ASSA ABLOY AB Class B 6,982,707.69 0.9 Vestas Wind Systems A/S 6,965,518.08 0.9 Merck KGaA 6,868,081.50 0.9 Iberdrola SA 6,581,084.07 0.8 Compagnie Generale des Etablissements Michelin SCA 6,555,056.14 0.8 Straumann Holding AG 6,480,282.66 0.8 Atlas Copco AB Class B 6,194,910.19 0.8 Deutsche Boerse AG 6,186,305.10 0.8 UPM-Kymmene Oyj 5,956,283.07 0.7 Deutsche Post AG 5,851,177.11 0.7 Enel SpA 5,808,234.13 0.7 AXA SA 5,790,969.55 0.7 Nintendo Co., Ltd. -
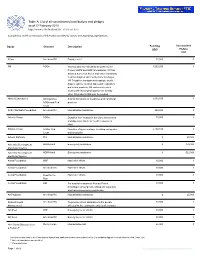
Table A: List of All Commitments/Contributions and Pledges As of 17 February 2010 (Table Ref: R10)
Table A: List of all commitments/contributions and pledges as of 17 February 2010 http://www.reliefweb.int/fts (Table ref: R10) Compiled by OCHA on the basis of information provided by donors and appealing organizations. Donor Channel Description Funding Uncommitted USD Pledges USD 3Com American RC Disaster relief 10,000 0 3M NGOs Working with key humanitarian partners like 1,000,000 0 Project HOPE and MAP International, 3M has donated numerous boxes and cases containing medical supplies such as Nexcare bandages, 3M Tegaderm transparent dressings, sterile drapes, splints, medical tapes and respiratory protection products. 3M continues to work closely with its nonprofit partners to identify other 3M products that may be needed. Abbott Laboratories UN Agencies, In-kind: Donations of medicines and nutritional 1,000,000 0 NGOs and Red products Cross ACE Charitable Foundation American RC Humanitarian assistance 250,000 0 Actavis Group NGOs Donation from Actavis in the US to Americares 10,000 0 and Operation Smile for health response in Haiti. Actavis Group NGOs; Red Donation of generic drugs, including analgesics 2,100,840 0 Cross and antibiotics. Advent Software PIH Humanitarian assistance 0 25,000 Adventist Development ADRA-Haiti Emergency assistance 0 478,000 and Relief Agency Adventist Development ADRA-Haiti Emergency assistance 0 522,000 and Relief Agency Aetna Foundation MSF Haiti relief efforts 10,000 0 Aetna Foundation American RC Haiti relief efforts 10,000 0 Aetna Foundation Food for the Haiti relief efforts 10,000 0 Poor Aetna Foundation UM For medical missions to Port-au-Prince, 10,000 0 including neurosurgeons, orthopedic surgeons and trauma/emergency physicians. -

Integrated Report 2016 Year Ended March 31, 2016 Strengths That Increase Supporting Sustainable Creating Long-Term Value Our Value to Society Manufacturing Processes
Integrated Report 2016 Year ended March 31, 2016 Strengths that increase Supporting sustainable Creating long-term value our value to society manufacturing processes The Company Policy of Shionogi Contents Strengths that increase our value to society The Company Policy of Shionogi (Established in 1957) Creating long-term value Supporting sustainable manufacturing processes Data Section Editorial Policy This Integrated Report provides a wide range of information to give shareholders, Forward-looking Statements investors and other stakeholders a deeper understanding of the Shionogi Group’s This report contains forward-looking statements. These statements are based on corporate value. In addition to financial data, readers can access information expectations in light of the information currently available, assumptions that are about management strategy and the Group’s governance, social and environmen- subject to risks, and uncertainties which could cause actual results to differ tal activities. materially from these statements. Risks and uncertainties include general domestic and international economic Period Under Review conditions, such as general industry and market conditions, and changes of Fiscal 2015 (April 1, 2015–March 31, 2016) interest rates and currency exchange rates. Certain activities continuing after fiscal 2015 are also included. These risks and uncertainties particularly apply to forward-looking statements concerning existing products and those under development. Product risks and Scope and Organization uncertainties include, but are not limited to, completion and discontinuation of This Integrated Report encompasses the activities of Shionogi & Co., Ltd. and 37 clinical trials; obtaining regulatory approvals; claims and concerns about product consolidated subsidiaries. safety and efficacy; technological advances; adverse outcome of important In this report, “Shionogi” refers to Shionogi & Co., Ltd. -

Translation for Reference Only Ticker Code: 4507 to All Shareholders June 5, 2018
Translation for reference only Ticker Code: 4507 To All Shareholders June 5, 2018 Notice of Convocation of the 153rd Annual General Meeting of Shareholders The 153rd Annual General Meeting of Shareholders will be convened at the time and location listed below. On behalf of the directors of the Company, we cordially invite you to attend this shareholders’ meeting. If you are unable to attend, you can exercise your voting rights with the proxy form on the back of this notice. If you wish to vote by using the proxy form, you are kindly requested to take the time to review the reference information provided below and exercise it by 5:00 p.m., Tuesday June 19, 2018.1 Yours faithfully, Isao Teshirogi Representative director and president and CEO Shionogi & Co., Ltd. 1-8, Doshomachi 3-chome, Chuo-ku, Osaka 541-0045, Japan Annual General Meeting of Shareholders 1. Date and time: 10:00 a.m., Wednesday, June 20, 2018 2. Location: HERBIS HALL 5-25, Umeda 2-chome, Kita-ku, Osaka 530-0001, Japan 3. Agenda: Items to report: 1. The Business Report, the Consolidated Financial Statements and the Non-Consolidated Financial Statements for the 153rd Fiscal Term (year ended March 31, 2018) 2. The Audit Report of the Consolidated Financial Statements for the 153rd Fiscal Term (year ended March 31, 2018) by the Accounting Auditor and the Board of Corporate Auditors Items for resolution: Proposal No. 1: Appropriation of Surplus Proposal No. 2: Amendments to the Articles of Incorporation Proposal No. 3: Election of Six (6) Directors Proposal No. -

Notice of Convocation of the 151St Annual General Meeting of Shareholders
Translation for reference only Ticker Code: 4507 To All Shareholders June 1, 2016 Notice of Convocation of the 151st Annual General Meeting of Shareholders The 151st Annual General Meeting of Shareholders will be convened at the time and location listed below. On behalf of the directors of the Company, we cordially invite you to attend this shareholders’ meeting. If you are unable to attend, you can exercise your voting rights with the proxy form on the back of this notice. If you wish to vote by using the proxy form, you are kindly requested to take the time to review the reference information provided below and exercise it by 5:00 p.m., Wednesday June 22, 2016.1 Yours faithfully, Isao Teshirogi Representative director and president and CEO Shionogi & Co., Ltd. 1-8, Doshomachi 3-chome, Chuo-ku, Osaka 541-0045, Japan Annual General Meeting of Shareholders 1. Date and time: 10:00 a.m., Thursday, June 23, 2016 2. Location: HERBIS HALL 5-25, Umeda 2-chome, Kita-ku, Osaka 530-0001, Japan 3. Agenda: Items to report: 1. The Business Report, the Consolidated Financial Statements and the Non-Consolidated Financial Statements for the 151st Fiscal Term (year ended March 31, 2016) 2. The Audit Report of the Consolidated Financial Statements for the 151st Fiscal Term (year ended March 31, 2016) by the Accounting Auditor and the Board of Corporate Auditors Items for resolution: Proposal No. 1: Appropriation of Surplus Proposal No. 2: Election of Six (6) Directors Proposal No. 3: Election of Two (2) Corporate Auditors 4. Exercise of voting rights: You are kindly requested to review “How to Exercise Your Voting Rights” on pages 2 and 3 before exercising your voting rights. -

Effect of Aspirin on Cancer Chemoprevention
Diabetes Care Volume 41, August 2018 1757 Effect of Aspirin on Cancer Sadanori Okada,1,2 Takeshi Morimoto,3 Hisao Ogawa,4 Mio Sakuma,3 Chemoprevention in Japanese Chisa Matsumoto,3 Hirofumi Soejima,5 Masafumi Nakayama,6 Naofumi Doi,7 Patients With Type 2 Diabetes: Hideaki Jinnouchi,8 Masako Waki,9 Izuru Masuda,10 and Yoshihiko Saito,2 for 10-Year Observational Follow-up the JPAD Trial Investigators* of a Randomized Controlled Trial Diabetes Care 2018;41:1757–1764 | https://doi.org/10.2337/dc18-0368 OBJECTIVE This study analyzed the efficacy of low-dose aspirin in cancer chemoprevention in patients with diabetes. 1Department of Diabetology, Nara Medical Uni- RESEARCH DESIGN AND METHODS versity, Kashihara, Nara, Japan 2 This study was a posttrial follow-up of the Japanese Primary Prevention of Department of Cardiovascular Medicine, Nara Medical University, Kashihara, Nara, Japan Atherosclerosis with Aspirin for Diabetes (JPAD) trial. Participants in the JPAD 3Department of Clinical Epidemiology, Hyogo trial (2,536 Japanese patients with type 2 diabetes and without preexisting College of Medicine, Nishinomiya, Hyogo, Japan 4 cardiovascular disease) were randomly allocated to receive aspirin (81 or 100 mg National Cerebral and Cardiovascular Center, PATHOPHYSIOLOGY/COMPLICATIONS daily) or no aspirin. After that trial ended in 2008, we followed up with the Osaka, Japan 5Department of Cardiology, Graduate School of participants until 2015, with no attempt to change the previously assigned ther- Medical Science, Kumamoto University, Chuo, apy. The primary end point was total cancer incidence. We investigated the effect Kumamoto, Japan of low-dose aspirin on cancer incidence. 6Nakayama Cardiovascular Clinic, Amakusa, Kumamoto, Japan RESULTS 7Department of Cardiovascular Medicine, Nara Prefectural Seiwa Medical Center, Sango, Ikoma, During the median follow-up period of 10.7 years, a total of 318 cancers occurred. -

Comprehensive List of Pharmaceuticals-Related Projects
Pharmaceutical and R&D Facilities Facility Type Photo Client Location Completion Scope Wakayama/ Solid Dosage TAMURA PHARMACEUTICAL CO.,LTD. 2019 EPCV Japan Gunma/ Bio Bulk DAIICHI SANKYO CHEMICAL PHARMA CO., LTD. 2019 EPCV Japan Hyogo/ R&D Center KANAE CO.,LTD. 2019 EPC Japan API undisclosed Tohoku/Japan 2017 EPCV R&D Center JSR Corporation Tokyo/Japan 2017 EPCV API undisclosed Kitakanto/Japan 2017 EPCV Yamaguchi/ R&D Center TEIJIN PHARMA LIMITED 2015 EPCV Japan Medical Device Menicon Co., Ltd. Chubu/Japan 2014 EPCV Fukushima/ R&D Center SANWA KAGAKU KENKYUSHO CO., LTD. 2014 EPCV Japan Yamaguchi/ Medical Device Terumo Yamaguchi Corporation 2014 E+ES Japan TOYAMA CHEMICAL CO., LTD. Toyama/ Parenteral 2013 EPCV (Current name:FUJIFILM Toyama Chemical Co., Ltd.) Japan Astellas Pharma Inc. Aichi/ Bio Bulk 2013 EPCV (Current name:Micro Biopharm Japan Co.,Ltd.) Japan Kanagawa/ R&D Center Mitsubishi Tanabe Pharma Corporation 2011 EPC Japan Hiroshima/ R&D Center YASUHARA CHEMICAL CO., LTD. 2010 EPC Japan Fukushima/ API TOA EIYO LTD. 2009 EPCV Japan Gunma/ R&D Center SANDEN HOLDINGS CORPORATION 2008 EPC Japan Hyogo/ Packaging KANAE CO., LTD. 2008 EPCV Japan ASAHI GLASS CO., LTD. Chiba/ Bio Bulk 2008 EPCV (Current name:AGC Inc.) Japan 1 / 3 Iwate/ Aseptic API Shionogi & Co., Ltd. 2007 EPCV Japan Mie/ Solid Dosage Sumitomo Dainippon Pharma Co., Ltd. 2007 EPCV Japan Saitama/ Nutrition/Liquid Taisho Pharmaceutical Co., Ltd. 2007 EPCV Japan Kaketsuken Kumamoto/ Vaccine (The Chemo-sero-therapeutic Research Institute) 2006 EPCV Japan (Current name:KM Biologics Co., Ltd.) Niigata/ Vaccine Denka Seiken Co., Ltd. 2006 EPCV Japan Shizuoka/ Bio Bulk Yamaha Motor Co., Ltd. -

Abbott Labora Tories Zambon Actelion Wockhardt Ajinomoto W a Tson Pharmaceuticals Alexion Pharmaceuticals W Arner Chilcott Aller
ZAMBON ABBOTT LABORATORIES WOCKHARDT ACTELION WATSON PHARMACEUTICALS AJINOMOTO WARNER CHILCOTT ALEXION PHARMACEUTICALS VERTEX PHARMACEUTICALS ALLERGAN VALEANT PHARMACEUTICALS ALMIRALL UNITED THERAPEUTICS AMGEN UCB AMYLIN PHARMACEUTICALS TORAY INDUSTRIES ASAHI KASEI TEVA ASPEN PHARMACARE TEIJIN ASTELLAS TAKEDA ASTRAZENECA TAISHO PHARMACEUTICAL BAXTER INTERNATIONAL SUN PHARMACEUTICAL INDUSTRIES BAYER STADA BIOGEN IDEC SIGMA-TAU BOEHRINGER INGELHEIM SHIRE BRISTOL-MYERS SQUIBB SHIONOGI CADILA SERVIER CELGENE SAWAI PHARMACEUTICAL CHIESI SANTEN CIPLA SANOFI COVIDIEN ROCHE CSL RECORDATI CUBIST PHARMACEUTICALS PIERRE FABRE DAIICHI SANKYO PHARMSTANDARD DAINIPPON SUMITOMO PFIZER DONG-A PAR PHARMACEUTICAL COMPANIES dr reddy’s OTSUKA HOLDINGS EISAI ORION PHARMA ELAN ONO PHARMACEUTICAL ENDO PHARMACEUTICALS NOVO NORDISK ESTEVE NOVARTIS FERRING NIPPON SHINYAKU FOREST LABORATORIES MYLAN GALDERMA MOCHIDA GALENICA AG MITSUBISHI TANABE GEDEON RICHTER 100 MERZ GILEAD SCIENCES MERCK KGAA GLAXOSMITHKLINE MERCK & CO GREEN CROSS MENARINI GRUENENTHAL MEIJI HOLDINGS HISAMITSU MEDICIS PHARMACEUTICAL HOSPIRA MEDA IPSEN MARUHO JAPAN TOBACCO LUPIN JOHNSON & JOHNSON LUNDBECK KAKEN PHARMACEUTICAL LILLY KISSEI LEO PHARMA KRKA KYOWA HAKKO KIRIN KYORIN HOLDINGS Pharma&Biotech Looking for an Easier Way? Bridge the Gap with Lonza’s Easy Access Services for ADCs Lonza offers early development and manufacturing services for your antibody-drug conjugates (ADCs). Take advantage of the best characteristics of monoclonal antibodies (mAbs) and the potency of cytotoxic molecules -

Going Dutch: Which Firms Are Moving to the Netherlands Because of Brexit?
SEPTEMBER 2019 Going Dutch: Which firms are Alert Clingendael moving to the Netherlands because of Brexit? Rem KortewegRem Continued uncertainty about Brexit has led setting up EU offices alongside their UK many UK-based businesses to activate their ones, deferring investments from UK contingency plans. Companies have decided sites to European plants, or relocating to move all, or part, of their operations to altogether. On 1 February 2019, the EU member-states in order to continue Institute of Directors – a British business to serve their customers in the single association – reported that, based on its market. Precautionary measures include survey of 1200 company directors, “nearly Clingendael Alert one in three UK firms” were planning for a off announcing their plans until Brexit has Brexit relocation.1 happened. Our list, however, mentions those companies that have already moved, or The Netherlands is one of the countries are in the process of moving. The list also firms have put on their shortlist. In late includes a number of firms which have January, the Dutch government’s foreign announced a move to the Netherlands in investment arm, NFIA, announced that it the event the UK leaves the EU without was talking to more than 250 companies a deal, but for obvious reasons have not which were thinking of moving some, or yet enacted those plans. It is also the first all, of their activities to the Netherlands.2 publicly available list of its kind for the Of course, any prudent executive will Netherlands. shop around and compare what Dutch regulators and tax authorities have to The Netherlands of course is not the only offer over their Irish, German, Spanish or destination for UK-based companies French peers. -

TSHP Virtual Seminar 2020 Partner, Supporter, and Exhibitor Listing
TSHP Virtual Seminar 2020 Partner, Supporter, and Exhibitor Listing Abbvie https://www.abbvie.com/ ___________________________________________________________________________________ AcelRx Pharmaceuticals http://www.acelrx.com AcelRx Pharmaceuticals, Inc. is a specialty pharmaceutical company focused on the development and commercialization of innovative therapies for use in medically supervised settings. In November of 2018, its lead product candidate DSUVIA™ (sufentanil sublingual tablet 30 mcg) was approved by the U.S. Food and Drug Administration. ___________________________________________________________________________________ AIS Healthcare http://www.aiscaregroup.com The leading 503A specialty pharmacy and targeted drug delivery care provider, AIS Healthcare is driven to improve the everyday lives of patients and providers, in ways big and small. From higher quality and sterility standards to services that improve care delivery, we do what’s right, not just what’s expected— every time. ___________________________________________________________________________________ Allergan http://www.allergan.com Allergan is a world leader in neuromodulator therapy and neurosciences. For over three decades, we have been committed to the research and clinical development of BOTOX® (OnabotulinumtoxinA), one of the world’s most versatile medicines, to improve the physical well-being and resulting quality of life for people around the world who suffer from a variety of serious or debilitating disorders. BOTOX® is available in more than 75 countries with 20 approved indications. ___________________________________________________________________________________ AMAG Pharmaceuticals http://www.amagpharma.com AMAG is a pharmaceutical company focused on bringing innovative products to patients with unmet medical needs. Every day the people at AMAG aim higher, devoting our passion and perseverance to finding new and better ways to support the health of patients and families. For additional company information, please visit www.amagpharma.com. -

Aberdeen Global Japanese Equity Fund a This Fund Is Managed by Aberdeen Standard Investments Luxembourg S.A
04 February, 2019 Aberdeen Global Japanese Equity Fund A This fund is managed by Aberdeen Standard Investments Luxembourg S.A. EFC Classification Equity Japan Price +/- Date 52wk range 430.92 EUR 3.01 01/02/2019 405.90 537.20 Issuer Profile Administrator Aberdeen Standard Investments Luxembourg S.A. The Fund's investment objective is long-term total return to be achieved by investing at Address 35a, avenue John F. Kennedy L- 1246 least two-thirds of the Fund's assets in equities and equity-related securities of companies with their registered office in Japan City Luxemburg Tel/Fax +352 2643 3000 Website www.aberdeen-asset.com Chart 5 year General Information ISIN LU0011963674 Fund Type Capitalization 550 Quote Frequency daily Quote Currency JPY Currency JPY 500 Foundation Date 26/04/1988 Fund Manager Asien Equity Team 450 Legal Type Investment company according to Luxembourg law UCITS Yes 400 Financial Year End 30/09/2018 Fund size 16,972,893,256.03 JPY Minimal Order 1,500.00 JPY 350 Costs 300 Entry fee 6.38 % 2015 2016 2017 2018 2019 Exit fee 0.00 % vwdgroup: Operation costs 1.50 % 38 Days 200 Days Ongoing charges 1.67 % Fund Returns 2014 2015 2016 2017 2018 2019 Returns 14.72 22.91 5.21 8.21 -17.28 4.45 Category Average 6.93 67.19 4.20 1966181.09 -13.20 5.36 Category Ranking 29 / 392 153 / 677 473 / 1004 912 / 1104 873 / 1140 940 / 1198 2,500k 2,000k 1,500k s e u l 1,000k a V 500k 0k -500k 2014 2015 2016 2017 2018 2019 Returns Category Average Fund Ratios (end previous month) Timing YTD 1 month 6 months 1 year 3 year 5 year Performance Aberdeen Global Japanese Equity Fund A 2.95 % 2.95 % -16.56 % -21.45 % 0.28 % 4.80 % Volatility Aberdeen Global Japanese Equity Fund A 16.13 % 11.77 % 15.24 % 04 February, 2019 Aberdeen Global Japanese Equity Fund A This fund is managed by Aberdeen Standard Investments Luxembourg S.A.