What Is Memory-Guided Attention? How Past Experiences Shape Selective Visuospatial Attention in the Present
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Semantic Memory - Psychology - Oxford Bibliographies
1/16/2019 Semantic Memory - Psychology - Oxford Bibliographies Semantic Memory Michael N. Jones, Johnathan Avery LAST MODIFIED: 15 JANUARY 2019 DOI: 10.1093/OBO/97801998283400231 Introduction Semantic memory refers to our general world knowledge that encompasses memory for concepts, facts, and the meanings of words and other symbolic units that constitute formal communication systems such as language or math. In the classic hierarchical view of memory, declarative memory was subdivided into two independent modules: episodic memory, which is our autobiographical store of individual events, and semantic memory, which is our general store of abstracted knowledge. However, more recent theoretical accounts have greatly reduced the independence of these two memory systems, and episodic memory is typically viewed as a gateway to semantic memory accessed through the process of abstraction. Modern accounts view semantic memory as deeply rooted in sensorimotor experience, abstracted across many episodic memories to highlight the stable characteristics and mute the idiosyncratic ones. A great deal of research in neuroscience has focused on both how the brain creates semantic memories and what brain regions share the responsibility for storage and retrieval of semantic knowledge. These include many classic experiments that studied the behavior of individuals with brain damage and various types of semantic disorders but also more modern studies that employ neuroimaging techniques to study how the brain creates and stores semantic memories. Classically, semantic memory had been treated as a miscellaneous area of study for anything in declarative memory that was not clearly within the realm of episodic memory, and formal models of meaning in memory did not advance at the pace of models of episodic memory. -

How Trauma Impacts Four Different Types of Memory
How Trauma Impacts Four Different Types of Memory EXPLICIT MEMORY IMPLICIT MEMORY SEMANTIC MEMORY EPISODIC MEMORY EMOTIONAL MEMORY PROCEDURAL MEMORY What It Is What It Is What It Is What It Is The memory of general knowledge and The autobiographical memory of an event The memory of the emotions you felt The memory of how to perform a facts. or experience – including the who, what, during an experience. common task without actively thinking and where. Example Example Example Example You remember what a bicycle is. You remember who was there and what When a wave of shame or anxiety grabs You can ride a bicycle automatically, with- street you were on when you fell off your you the next time you see your bicycle out having to stop and recall how it’s bicycle in front of a crowd. after the big fall. done. How Trauma Can Affect It How Trauma Can Affect It How Trauma Can Affect It How Trauma Can Affect It Trauma can prevent information (like Trauma can shutdown episodic memory After trauma, a person may get triggered Trauma can change patterns of words, images, sounds, etc.) from differ- and fragment the sequence of events. and experience painful emotions, often procedural memory. For example, a ent parts of the brain from combining to without context. person might tense up and unconsciously make a semantic memory. alter their posture, which could lead to pain or even numbness. Related Brain Area Related Brain Area Related Brain Area Related Brain Area The temporal lobe and inferior parietal The hippocampus is responsible for The amygdala plays a key role in The striatum is associated with producing cortex collect information from different creating and recalling episodic memory. -

PSYC20006 Notes
PSYC20006 BIOLOGICAL PSYCHOLOGY PSYC20006 1 COGNITIVE THEORIES OF MEMORY Procedural Memory: The storage of skills & procedures, key in motor performance. It involves memory systems that are independent of the hippocampal formation, in particular, the cerebellum, basal ganglia, cortical motor sites. Doesn't involve mesial-temporal function, basal forebrain or diencephalon. Declarative memory: Accumulation of facts/data from learning experiences. • Associated with encoding & maintaining information, which comes from higher systems in the brain that have processed the information • Information is then passed to hippocampal formation, which does the encoding for elaboration & retention. Hippocampus is in charge of structuring our memories in a relational way so everything relating to the same topic is organized within the same network. This is also how memories are retrieved. Activation of 1 piece of information will link up the whole network of related pieces of information. Memories are placed into an already exiting framework, and so memory activation can be independent of the environment. MODELS OF MEMORY Serial models of Memory include the Atkinson-Shiffrin Model, Levels of Processing Model & Tulving’s Model — all suggest that memory is processed in a sequential way. A parallel model of memory, the Parallel Distributed Processing Model, is one which suggests types of memories are processed independently. Atkinson-Shiffrin Model First starts as Sensory Memory (visual / auditory). If nothing is done with it, fades very quickly but if you pay attention to it, it will move into working memory. Working Memory contains both new information & from long-term memory. If it goes through an encoding process, it will be in long-term memory. -

Semantic Priming in Schizophrenia: a Review and Synthesis
Journal of the International Neuropsychological Society (2002), 8, 699–720. Copyright © 2002 INS. Published by Cambridge University Press. Printed in the USA. DOI: 10.1017.S1355617702801357 CRITICAL REVIEW Semantic priming in schizophrenia: A review and synthesis MICHAEL J. MINZENBERG,1 BETH A. OBER,2 and SOPHIA VINOGRADOV1 1Department of Psychiatry, University of California, San Francisco and Department of Veterans Affairs Medical Center, San Francisco, California 2Department of Human and Community Development, University of California, Davis and Department of Veterans Affairs Northern California Health Care System, Martinez, California (Received October 9, 2000; Revised June 4, 2001; Accepted June 5, 2001) Abstract In this paper, we present a review of semantic priming experiments in schizophrenia. Semantic priming paradigms show utility in assessing the role of deficits in semantic memory network access in the pathology of schizophrenia. The studies are placed in the context of current models of information processing. In this review we include all English-language reports (from peer-reviewed journals) of single-word semantic priming studies involving participants with schizophrenia. The studies to date show schizophrenic patients to exhibit variable semantic priming effects under automatic processing conditions, and consistent impairments under controlled0attentional conditions. We also describe associations with other neurocognitive dysfunction, neurochemical and electrophysiological disturbances, and clinical manifestations (such as thought disorder). (JINS, 2002, 8, 699–720.) Keywords: Semantic priming, Schizophrenia, Semantic memory, Language, Information processing INTRODUCTION Semantic Memory and Spreading Schizophrenia is primarily a disorder of thinking and lan- Activation Network Models guage. Indeed, investigators have suggested that a defect in All information processing models posit the existence of a language information processing may be pathognomonic of long-term memory system. -

Memory & Cognition
/ Memory & Cognition Volume 47 · Number 4 · May 2019 Special Issue: Recognizing Five Decades of Cumulative The role of control processes in temporal Progress in Understanding Human Memory and semantic contiguity and its Control Processes Inspired by Atkinson M.K. Healey · M.G. Uitvlugt 719 and Shiffrin (1968) Auditory distraction does more than disrupt rehearsal Guest Editors: Kenneth J. Malmberg· processes in children's serial recall Jeroen G. W. Raaijmakers ·Richard M. Shiffrin A.M. AuBuchon · C.l. McGill · E.M. Elliott 738 50 years of research sparked by Atkinson The effect of working memory maintenance and Shiffrin (1968) on long-term memory K.J. Malmberg · J.G.W. Raaijmakers · R.M. Shiffrin 561 J.K. Hartshorne· T. Makovski 749 · From ·short-term store to multicomponent working List-strength effects in older adults in recognition memory: The role of the modal model and free recall A.D. Baddeley · G.J. Hitch · R.J. Allen 575 L. Sahakyan 764 Central tendency representation and exemplar Verbal and spatial acquisition as a function of distributed matching in visual short-term memory practice and code-specific interference C. Dube 589 A.P. Young· A.F. Healy· M. Jones· L.E. Bourne Jr. 779 Item repetition and retrieval processes in cued recall: Dissociating visuo-spatial and verbal working memory: Analysis of recall-latency distributions It's all in the features Y. Jang · H. Lee 792 ~1 . Poirier· J.M. Yearsley · J. Saint-Aubin· C. Fortin· G. Gallant · D. Guitard 603 Testing the primary and convergent retrieval model of recall: Recall practice produces faster recall Interpolated retrieval effects on list isolation: success but also faster recall failure IndiYiduaLdifferences in working memory capacity W.J. -

Models of Memory
To be published in H. Pashler & D. Medin (Eds.), Stevens’ Handbook of Experimental Psychology, Third Edition, Volume 2: Memory and Cognitive Processes. New York: John Wiley & Sons, Inc.. MODELS OF MEMORY Jeroen G.W. Raaijmakers Richard M. Shiffrin University of Amsterdam Indiana University Introduction Sciences tend to evolve in a direction that introduces greater emphasis on formal theorizing. Psychology generally, and the study of memory in particular, have followed this prescription: The memory field has seen a continuing introduction of mathematical and formal computer simulation models, today reaching the point where modeling is an integral part of the field rather than an esoteric newcomer. Thus anything resembling a comprehensive treatment of memory models would in effect turn into a review of the field of memory research, and considerably exceed the scope of this chapter. We shall deal with this problem by covering selected approaches that introduce some of the main themes that have characterized model development. This selective coverage will emphasize our own work perhaps somewhat more than would have been the case for other authors, but we are far more familiar with our models than some of the alternatives, and we believe they provide good examples of the themes that we wish to highlight. The earliest attempts to apply mathematical modeling to memory probably date back to the late 19th century when pioneers such as Ebbinghaus and Thorndike started to collect empirical data on learning and memory. Given the obvious regularities of learning and forgetting curves, it is not surprising that the question was asked whether these regularities could be captured by mathematical functions. -

Okami Study Guide: Chapter 8 1
Okami Study Guide: Chapter 8 1 Chapter in Review 1. Memory may be defined as a group of mechanisms and systems that encode, store, and retrieve information. The modal model of memory describes three stages and stores in the memory process: sensory memory, short-term memory (STM), and long- term memory (LTM). 2. Sensory memory very briefly stores fleeing sensory impressions for further processing in STM and LTM. Sensory memory is divided into two categories: iconic store, which stores fleeting visual impressions; and echoic store, which stores fleeting auditory impressions. In addition to storing sensory impressions for further processing, sensory memory allows us to perceive the world as a continuous stream of events instead of a series of “snapshots.” 3. When you consciously or unconsciously decide to pay attention to specific pieces of information in sensory memory, the information is transferred into short-term memory. The duration and capacity of STM are limited. In general, information can remain in STM for no longer than 20 seconds unless maintenance rehearsal takes place, and no more than 4 single items or chunks of information can be held in STM at any one time. A chunk is any grouping of items that are strongly associated with one another. 4. Long-term memory (LTM) is theoretically limitless and relatively permanent. Information moves from STM to LTM when it is encoded in one of three ways: through sound (acoustic encoding), imagery (visual encoding), or meaning (semantic encoding). Encoding in STM tends to be primarily acoustic, secondarily visual, and much less often semantic. However, encoding in LTM is most effective if it is semantic. -
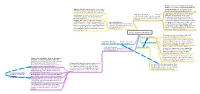
Long Term Memory & Amnesia
Previous theories of Amnesia: encoding faliure (consolidation) rapid forgetting and Amnesic Patients typically retain old procedural retrieval failure (retroactive/proactive information and are reasonably good at learning interference, Underwood; McGeoch) new procedural skills (H.M and mirror writing) Important Current Theory: Contextual Amnesia & the Brain Linked to Conditioning: eyeblink with puf of air leads to Memory Theory (Ryan et al. 2000) medial temporal lobe and diencephalic conditioned response. Patients with amnesia impairment in integrating of binding region, including mamillary bodies and typically acquire this. contextual/relational features of memory. thalamus. Priming: Improvement or bias in performance The medial temporal lobe and hippocampus resulting from prior, supraliminal presentation of Procedural Memory are proposed to bind events to the contexts stimuli. Tulving et al. (1982) primed participants Often relatively automatic processes in which they occur. This bypasses the with a list of words and then showed them letters (requiring little attention) allowing for declarative vs procedural distinction. And (cued-recall). Recall was better for words behavioural responses to there is reasonable support for this theory presented earlier. Amnesic patients are relatively environmental cues. (Channon, Shanks et al 2006.) normal on these tasks. Long-Term Memory & Amnesia Temporary storage of information with rapid Short-Term Memory decay and sensitivity to interference. Supported by studies of recency efect (Murdock, Postman), which is taken as evidence of short Long-Term Memory Mediates declarative Double Dissociation?! but not non-declarative (procedural) memory. term memory store. Also by studies supporting subvocal speech (Baddeley, 1966) However, studies have provided evidence of Declarative Memory This is knowledge STM link with LTM retrieved by explicit, deliberate recollection. -

Encoding and Retrieval from Long-Term Memory
SMITMC05_0131825089.QXD 3/29/06 12:49 AM Page 192 REVISED PAGES CHAPTER 5 Encoding and Retrieval from Long-Term Memory Learning Objectives 1. The Nature of Long-Term Memory 3.3. Cues for Retrieval 1.1. The Forms of Long-Term Memory 3.4. The Second Time Around: Recognizing 1.2. The Power of Memory: The Story of H.M. Stimuli by Recollection and Familiarity 1.3. Multiple Systems for Long-Term DEBATE: “Remembering,” “Knowing,” and Learning and Remembering the Medial Temporal Lobes 2. Encoding: How Episodic Memories are 3.5. Misremembering the Past Formed 3.5.1. Bias 2.1. The Importance of Attention 3.5.2. Misattribution A CLOSER LOOK: Transfer Appropriate 3.5.3. Suggestion Processing 4. The Encoding Was Successful, But I Still 2.2. Levels of Processing and Elaborative Can’t Remember Encoding 4.1. Ebbinghaus’s Forgetting Function 2.2.1. Levels-of-Processing Theory: 4.2. Forgetting and Competition Argument and Limitations 4.2.1. Retroactive and Proactive 2.2.2. The Brain, Semantic Elabora- Interference tion, and Episodic Encoding 4.2.2. Blocking and Suppression 2.3. Enhancers of Encoding: Generation and 5. Nondeclarative Memory Systems Spacing 5.1. Priming 2.3.1. The Generation Effect 5.1.1. Perceptual Priming 2.3.2. The Spacing Effect 5.1.2. Conceptual Priming 2.4. Episodic Encoding, Binding, and the 5.2. Beyond Priming: Other Forms of Non- Medial Temporal Lobe declarative Memory 2.5. Consolidation: The Fixing of Memory 5.2.1. Skill Learning 3. Retrieval: How We Recall the Past from 5.2.2. -

Neuroimaging Studies of Semantic Memory: Inferring “How” from “Where” Sharon L
Neuropsychologia 41 (2003) 280–292 Neuroimaging studies of semantic memory: inferring “how” from “where” Sharon L. Thompson-Schill∗ Department of Psychology, University of Pennsylvania, 3815 Walnut Street, Philadelphia, PA 19104-6196, USA Abstract For nearly two decades, functional neuroimaging studies have attempted to shed light on questions about the representation, organization, and retrieval of semantic knowledge. This review examines some of the major findings in this area. For example, functional neuroimaging studies have examined the extent to which there is a unitary semantic system or a series of multiple semantic subsystems organized by input modality, knowledge attribute, and/or taxonomic category. Additionally, functional neuroimaging studies have investigated the contributions of frontal cortex to semantic retrieval and selection. Collectively, these studies demonstrate that functional neuroimaging can offer more than neuroanatomical localization information; in addition, these studies offer new insights into longstanding questions about semantic memory. © 2002 Elsevier Science Ltd. All rights reserved. Keywords: Conceptual knowledge; fMRI; PET; Imagery; Selection; Frontal lobes 1. Introduction and structure of semantic networks (e.g. [19]). For many years, investigations of these and related topics were largely Tulving first made the distinction between our general conducted using classic behavioral methods, such as the ex- knowledge of concepts and facts, called semantic memory, amination of measurements of speed and accuracy in normal and our specific memory for personal experiences, called subjects, occasionally augmented by quantitative models of episodic memory [105]. The information that one ate eggs these phenomena and observations in patients with neuro- and toast for breakfast is an example of episodic memory, logical impairments. However, the past 15 years has seen whereas knowledge that eggs, toast, cereal, and pancakes are a resurgence of interest in the area of semantic memory, typical breakfast foods is an example of semantic memory. -

Semantic Priming in Mild Cognitive Impairment and Healthy Subjects: Effect of Different Time of Presentation of Word-Pairs
Journal of Personalized Medicine Article Semantic Priming in Mild Cognitive Impairment and Healthy Subjects: Effect of Different Time of Presentation of Word-Pairs Valeria Guglielmi 1, Davide Quaranta 1,*, Ilaria Mega 2, Emanuele Maria Costantini 2, Claudia Carrarini 2, Alice Innocenti 2 and Camillo Marra 2,3 1 Neurology Unit, Fondazione Policlinico Agostino Gemelli-IRCCS, 00168 Rome, Italy; [email protected] 2 Department of Neuroscience, Catholic University of Sacred Heart, 00168 Rome, Italy; [email protected] (I.M.); [email protected] (E.M.C.); [email protected] (C.C.); [email protected] (A.I.); [email protected] (C.M.) 3 Memory Clinic, Fondazione Policlinico Universitario Agostino Gemelli-IRCCS, 00168 Rome, Italy * Correspondence: [email protected] Received: 24 May 2020; Accepted: 25 June 2020; Published: 29 June 2020 Abstract: Introduction: Semantic memory is impaired in mild cognitive impairment (MCI). Two main hypotheses about this finding are debated and refer to the degradation of stored knowledge versus the impairment of semantic access mechanisms. The aim of our study is to evaluate semantic impairment in MCI versus healthy subjects (HS) by an experiment evaluating semantic priming. Methods: We enrolled 27 MCI and 20 HS. MCI group were divided, according to follow up, into converters-MCI and non converters-MCI. The semantic task consisted of 108 pairs of words, 54 of which were semantically associated. Stimuli were presented 250 or 900 ms later the appearance of the target in a randomized manner. Data were analyzed using factorial ANOVA. Results: Both HS and MCI answered more quickly for word than for non-word at both stimulus onset asynchrony (SOA) intervals. -

The Cognitive Neuroscience of Semantic Memory
To appear in the Oxford Handbook of Cognitive Neuroscience. Kevin Ochsner and Stephen Kosslyn (Eds.) Oxford University Press. The Cognitive Neuroscience of Semantic Memory Eiling Yee1, Evangelia G. Chrysikou2, and Sharon L. Thompson-Schill3 1Basque Center on Cognition, Brain and Language, San Sebastian, Spain; 2University of Kansas, Lawrence, KS, USA; 3University of Pennsylvania, Philadelphia, PA, USA Abstract Semantic memory refers to general knowledge about the world, including concepts, facts and beliefs (e.g., that a lemon is normally yellow and sour or that Paris is in France). How is this kind of knowledge acquired or lost? How is it stored and retrieved? In this chapter, we review evidence that conceptual knowledge about concrete objects is acquired through experience with them, distributed across brain regions that are involved in perceiving or acting upon them, and impaired via damage to these brain regions. We suggest that these distributed representations result in flexible concepts that can vary depending on the task and context, as well as on individual experience. Further, we discuss the role of brain regions implicated in selective attention in supporting such conceptual flexibility. Finally, we consider the neural bases of other aspects of conceptual knowledge, such as the ability to generalize (e.g., to map lemons and grapes onto the category fruit), and the ability to represent knowledge that does not have a direct sensorimotor correlate (e.g., abstract concepts, such as peace). Keywords semantic memory; concepts; categories; representation; knowledge; sensory-motor; grounding; embodiment 1. Introduction 1.2 What is the relationship between semantic and episodic memory? 1.1 What is semantic memory? Although semantic and episodic memory are typically How do we know what we know about the world? For considered distinct, the degree to which semantic memory is instance, how do we know that a cup must be concave, or that a dependent on episodic memory is a matter of ongoing debate.