Johnson 2011 Light Temperature Bletia Purpurea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bletilla Striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization
plants Article Bletilla striata (Orchidaceae) Seed Coat Restricts the Invasion of Fungal Hyphae at the Initial Stage of Fungal Colonization Chihiro Miura 1, Miharu Saisho 1, Takahiro Yagame 2, Masahide Yamato 3 and Hironori Kaminaka 1,* 1 Faculty of Agriculture, Tottori University, 4-101 Koyama Minami, Tottori 680-8553, Japan 2 Mizuho Kyo-do Museum, 316-5 Komagatafujiyama, Mizuho, Tokyo 190-1202, Japan 3 Faculty of Education, Chiba University, 1-33 Yayoicho, Inage-ku, Chiba 263-8522, Japan * Correspondence: [email protected]; Tel.: +81-857-31-5378 Received: 24 June 2019; Accepted: 8 August 2019; Published: 11 August 2019 Abstract: Orchids produce minute seeds that contain limited or no endosperm, and they must form an association with symbiotic fungi to obtain nutrients during germination and subsequent seedling growth under natural conditions. Orchids need to select an appropriate fungus among diverse soil fungi at the germination stage. However, there is limited understanding of the process by which orchids recruit fungal associates and initiate the symbiotic interaction. This study aimed to better understand this process by focusing on the seed coat, the first point of fungal attachment. Bletilla striata seeds, some with the seed coat removed, were prepared and sown with symbiotic fungi or with pathogenic fungi. The seed coat-stripped seeds inoculated with the symbiotic fungi showed a lower germination rate than the intact seeds, and proliferated fungal hyphae were observed inside and around the stripped seeds. Inoculation with the pathogenic fungi increased the infection rate in the seed coat-stripped seeds. The pathogenic fungal hyphae were arrested at the suspensor side of the intact seeds, whereas the seed coat-stripped seeds were subjected to severe infestation. -

Endophytic Colletotrichum Species from Bletilla Ochracea (Orchidaceae), with Descriptions of Seven New Speices
Fungal Diversity (2013) 61:139–164 DOI 10.1007/s13225-013-0254-5 Endophytic Colletotrichum species from Bletilla ochracea (Orchidaceae), with descriptions of seven new speices Gang Tao & Zuo-Yi Liu & Fang Liu & Ya-Hui Gao & Lei Cai Received: 20 May 2013 /Accepted: 1 July 2013 /Published online: 19 July 2013 # Mushroom Research Foundation 2013 Abstract Thirty-six strains of endophytic Colletotrichum ornamental plants and important research materials for coevo- species were isolated from leaves of Bletilla ochracea Schltr. lution between plants and fungi because of their special sym- (Orchidaceae) collected from 5 sites in Guizhou, China. biosis with mycorrhizal fungi (Zettler et al. 2004; Stark et al. Seventeen different species, including 7 new species (namely 2009; Nontachaiyapoom et al. 2010). Recently, the fungal C. bletillum, C. caudasporum, C. duyunensis, C. endophytum, communities in leaves and roots of orchid Bletilla ochracea C. excelsum-altitudum and C. guizhouensis and C. ochracea), have been investigated and the results indicated that there is a 8 previously described species (C. boninense, C. cereale, C. high diversity of endophytic fungi, including species from the destructivum, C. karstii, C. liriopes, C. miscanthi, C. genus Colletotrichum Corda (Tao et al. 2008, 2012). parsonsiae and C. tofieldiae) and 2 sterile mycelia were iden- Endophytic fungi live asymptomatically and internally with- tified. All of the taxa were identified based on morphology and in different tissues (e.g. leaves, roots) of host plants (Ganley phylogeny inferred from multi-locus sequences, including the and Newcombe 2006; Promputtha et al. 2007; Hoffman and nuclear ribosomal internal transcribed spacer (ITS) region, Arnold 2008). -
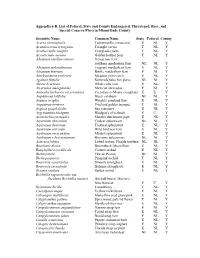
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Redalyc.EFFORTS to CONSERVE ENDANGERED TERRESTRIAL
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica RANGEL-VILLAFRANCO, MONICA; ORTEGA-LARROCEA, M. PILAR EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 326-333 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813068 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 326-333. 2007. EFFORTS TO CONSERVE ENDANGERED TERRESTRIAL ORCHIDS IN SITU AND EX SITU AT TWO NATURAL RESERVES WITHIN CENTRAL MEXICO 1 1,2 MONICA RANGEL-VILLAFRANCO & M. PILAR ORTEGA-LARROCEA 1 Departamento de Edafología, Instituto de Geología, Universidad Nacional Autónoma de México. Circuito Exterior de Ciudad Universitaria, México Distrito Federal, 04510. México. 2 Author for correspondence: [email protected] KEY WORDS: in situ conservation, ex situ conservation, orchid fungi isolation, seed banks The natural vegetation in and around Mexico City macrobulon, Epidendrum anisatu, Habenaria strictis- once harbored an unusually high number of plant and sima, Liparis greenwoodiana) (Hágsater et al. 2005). animal (insect) species, including endemics (Vázquez In contrast, in the Chichinautzin Area, eight types 1973, Ceballos & Galindo 1984, Rzedowski 1991). of vegetation can be found. An altitudinal gradient The high diversity in this region has been attributed joint with successive periods of volcanic activity to the unusual topography resulting from a series of are combined and pedogenetic processes through volcanic eruptions that ended ca. -

CITES Orchid Checklist Volumes 1, 2 & 3 Combined
CITES Orchid Checklist Online Version Volumes 1, 2 & 3 Combined (three volumes merged together as pdf files) Available at http://www.rbgkew.org.uk/data/cites.html Important: Please read the Introduction before reading this Part Introduction - OrchidIntro.pdf Part I : All names in current use - OrchidPartI.pdf (this file) Part II: Accepted names in current use - OrchidPartII.pdf Part III: Country Checklist - OrchidPartIII.pdf For the genera: Aerangis, Angraecum, Ascocentrum, Bletilla, Brassavola, Calanthe, Catasetum, Cattleya, Constantia, Cymbidium, Cypripedium, Dendrobium (selected sections only), Disa, Dracula, Encyclia, Laelia, Miltonia, Miltonioides, Miltoniopsis, Paphiopedilum, Paraphalaenopsis, Phalaenopsis, Phragmipedium, Pleione, Renanthera, Renantherella, Rhynchostylis, Rossioglossum, Sophronitella, Sophronitis Vanda and Vandopsis Compiled by: Jacqueline A Roberts, Lee R Allman, Sharon Anuku, Clive R Beale, Johanna C Benseler, Joanne Burdon, Richard W Butter, Kevin R Crook, Paul Mathew, H Noel McGough, Andrew Newman & Daniela C Zappi Assisted by a selected international panel of orchid experts Royal Botanic Gardens, Kew Copyright 2002 The Trustees of The Royal Botanic Gardens Kew CITES Secretariat Printed volumes: Volume 1 first published in 1995 - Volume 1: ISBN 0 947643 87 7 Volume 2 first published in 1997 - Volume 2: ISBN 1 900347 34 2 Volume 3 first published in 2001 - Volume 3: ISBN 1 84246 033 1 General editor of series: Jacqueline A Roberts 2 Part I: ORCHIDACEAE BINOMIALS IN CURRENT USAGE Ordered alphabetically on All -

A History of Orchids. a History of Discovery, Lust and Wealth
Scientific Papers. Series B, Horticulture. Vol. LXIV, No. 1, 2020 Print ISSN 2285-5653, CD-ROM ISSN 2285-5661, Online ISSN 2286-1580, ISSN-L 2285-5653 A HISTORY OF ORCHIDS. A HISTORY OF DISCOVERY, LUST AND WEALTH Nora Eugenia D. G. ANGHELESCU1, Annie BYGRAVE2, Mihaela I. GEORGESCU1, Sorina A. PETRA1, Florin TOMA1 1University of Agronomic Sciences and Veterinary Medicine of Bucharest, 59 Mărăști Blvd, District 1, Bucharest, Romania 2Self-employed, London, UK Corresponding author email: [email protected] Abstract Orchidaceae is the second largest families of flowering plants. There are approximately 900 orchid genera comprising between 28,000-32,000 species of orchids. The relationship between orchids and mankind is complex. The history of orchids’ discovery goes hand in hand with the history of humanity, encompassing discovery and adventure, witchcraft and magic, symbolism and occultism, addiction and sacrifice, lust and wealth. Historically, the Chinese were the first to cultivate orchids as medicinal plants, more than 4000 years ago. Gradually, records about orchids spread, reaching the Middle East and Europe. Around 300 B.C., Theophrastus named them for the first time orkhis. In 1737, Carl Linnaeus first used the word Orchidaceae to designate plants with similar features. The family name, Orchidaceae was fully established in 1789, by Antoine Laurent de Jussieu. In 1862, Charles Darwin published the first edition of his book, Fertilisation of Orchids. Darwin considered the adaptations of orchid flowers to their animal pollinators as being among the best examples of his idea of evolution through natural selection. Orchidology was on its way. During the 18th and the 19th centuries, orchids generated the notorious Orchid Fever where orchid-hunters turned the search for orchids into a frantic and obsessive hunt. -

PC22 Doc. 22.1 Annex (In English Only / Únicamente En Inglés / Seulement En Anglais)
Original language: English PC22 Doc. 22.1 Annex (in English only / únicamente en inglés / seulement en anglais) Quick scan of Orchidaceae species in European commerce as components of cosmetic, food and medicinal products Prepared by Josef A. Brinckmann Sebastopol, California, 95472 USA Commissioned by Federal Food Safety and Veterinary Office FSVO CITES Management Authorithy of Switzerland and Lichtenstein 2014 PC22 Doc 22.1 – p. 1 Contents Abbreviations and Acronyms ........................................................................................................................ 7 Executive Summary ...................................................................................................................................... 8 Information about the Databases Used ...................................................................................................... 11 1. Anoectochilus formosanus .................................................................................................................. 13 1.1. Countries of origin ................................................................................................................. 13 1.2. Commercially traded forms ................................................................................................... 13 1.2.1. Anoectochilus Formosanus Cell Culture Extract (CosIng) ............................................ 13 1.2.2. Anoectochilus Formosanus Extract (CosIng) ................................................................ 13 1.3. Selected finished -

Phylogeny, Character Evolution and the Systematics of Psilochilus (Triphoreae)
THE PRIMITIVE EPIDENDROIDEAE (ORCHIDACEAE): PHYLOGENY, CHARACTER EVOLUTION AND THE SYSTEMATICS OF PSILOCHILUS (TRIPHOREAE) A Dissertation Presented in Partial Fulfillment of the Requirements for The Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Erik Paul Rothacker, M.Sc. ***** The Ohio State University 2007 Doctoral Dissertation Committee: Approved by Dr. John V. Freudenstein, Adviser Dr. John Wenzel ________________________________ Dr. Andrea Wolfe Adviser Evolution, Ecology and Organismal Biology Graduate Program COPYRIGHT ERIK PAUL ROTHACKER 2007 ABSTRACT Considering the significance of the basal Epidendroideae in understanding patterns of morphological evolution within the subfamily, it is surprising that no fully resolved hypothesis of historical relationships has been presented for these orchids. This is the first study to improve both taxon and character sampling. The phylogenetic study of the basal Epidendroideae consisted of two components, molecular and morphological. A molecular phylogeny using three loci representing each of the plant genomes including gap characters is presented for the basal Epidendroideae. Here we find Neottieae sister to Palmorchis at the base of the Epidendroideae, followed by Triphoreae. Tropidieae and Sobralieae form a clade, however the relationship between these, Nervilieae and the advanced Epidendroids has not been resolved. A morphological matrix of 40 taxa and 30 characters was constructed and a phylogenetic analysis was performed. The results support many of the traditional views of tribal composition, but do not fully resolve relationships among many of the tribes. A robust hypothesis of relationships is presented based on the results of a total evidence analysis using three molecular loci, gap characters and morphology. Palmorchis is placed at the base of the tree, sister to Neottieae, followed successively by Triphoreae sister to Epipogium, then Sobralieae. -

Orchids in the Home by Heidi Napier UCCE Master Gardener of El Dorado County Orchids Have a Reputation for Being Difficult to Gr
August 10, 2016 Orchids in the Home By Heidi Napier UCCE Master Gardener of El Dorado County Orchids have a reputation for being difficult to grow, but many species do well in homes and in yards. There are more and more orchids available for purchase at grocery stores and nurseries, and most of these orchids do well under average home conditions, much like African Violets. Phaelanopsis, or Moth Orchid, is the most commonly sold for growing indoors. The flowers come in many colors -- white, yellow, purple, pink and even multicolor. These plants do well at indoor temperatures and the relatively low humidity found in most homes. Their natural bloom season is late winter to early spring, and the flowers may last one or two months. If you trim the spent flower stalk down to four to eight inches, it may rebloom. The main reasons many Phaelanopsis don’t rebloom are: 1. Not enough light. An east or south window or a skylight is good as long as the plant is protected from direct sun. 2. Too much water. The medium around the roots should dry out between watering or they will rot. Many orchids are sold in plastic or ceramic pots with no air circulation, and this promotes rotten roots. It is best to repot them in a plastic pot with slits in the side or into an unglazed ceramic pot. Most indoor orchids don’t grow in soil because in their natural habitat, they grow on trees, and their roots grow in the air. They often do best in a medium such as chunks of fir bark or coconut husk. -

Cryopreservation of Orchid Genetic Resources by Desiccation: a Case Study of Bletilla Formosana 203
Chapter 12 Provisional chapter Cryopreservation of Orchid Genetic Resources by Desiccation:Cryopreservation A Case of Study Orchid of Genetic Bletilla Resources formosana by Desiccation: A Case Study of Bletilla formosana Rung‐Yi Wu, Shao‐Yu Chang, Ting‐Fang Hsieh, Keng‐ChangRung-Yi Wu, Shao-YuChuang, Chang,Ie Ting, Ting-FangYen‐Hsu Lai Hsieh, and Keng-Chang Chuang, Le Ting, Yen-Hsu Lai, and Yu‐Sen Chang Yu-Sen Chang Additional information is available at the end of the chapter Additional information is available at the end of the chapter http://dx.doi.org/10.5772/65302 Abstract Many native orchid populations declined yearly due to economic development and climate change. This resulted in some wild orchids being threatened. In order to main- tain the orchid genetic resources, development of proper methods for the long-term preservation is urgent. Low temperature or dry storage methods for the preservation of orchid genetic resources have been implemented but are not effective in maintaining high viability of certain orchids for long periods. Cryopreservation is one of the most acceptable methods for long-term conservation of plant germplasm. Orchid seeds and pollens are ideal materials for long-term preservation (seed banking) in liquid nitrogen (LN) as the seeds and pollens are minute, enabling the storage of many hundreds of thousands of seeds or pollens in a small vial, and as most species germinate readily, making the technique very economical. This article describes cryopreservation of orchid genetic resources by desiccation and a case study of Bletilla formosana. We hope to provide a more practical potential cryopreservation method for future research needs. -

Optimizing the Extraction of Polysaccharides from Bletilla Ochracea Schltr. Using Response Surface Methodology (RSM) and Evaluating Their Antioxidant Activity
processes Article Optimizing the Extraction of Polysaccharides from Bletilla ochracea Schltr. Using Response Surface Methodology (RSM) and Evaluating their Antioxidant Activity Bulei Wang 1, Yan Xu 1, Lijun Chen 1, Guangming Zhao 1, Zeyuan Mi 1, Dinghao Lv 2 and Junfeng Niu 1,* 1 National Engineering Laboratory for Resource Development of Endangered Crude Drugs in Northwest China, The Key Laboratory of Medicinal Resources and Natural Pharmaceutical Chemistry, The Ministry of Education, College of Life Sciences, Shaanxi Normal University, Xi’an 710119, China; [email protected] (B.W.); [email protected] (Y.X.); [email protected] (L.C.); [email protected] (G.Z.); [email protected] (Z.M.) 2 Shanxi Institute of Medicine and Life Sciences, Taiyuan 030006, China; [email protected] * Correspondence: [email protected]; Tel.: +86-29-85310680 Received: 24 February 2020; Accepted: 10 March 2020; Published: 16 March 2020 Abstract: Bletilla ochracea Schltr. polysaccharides (BOP) have a similar structure to Bletilla striata (Thunb.) Reichb.f. (Orchidaceae) polysaccharides (BSP). Therefore, BOP can be considered as a substitute for BSP in the food, pharmaceuticals and cosmetics fields. To the best of our knowledge, little information is available regarding the optimization of extraction and antioxidant activity of BOP. In this study, response surface methodology (RSM) was firstly used for optimizing the extraction parameters of BOP. The results suggested that the optimal conditions included a temperature of 82 ◦C, a duration of 85 min and a liquid/material ratio of 30 mL/g. In these conditions, we received 26.45% 0.18% as the experimental yield. In addition, BOP exhibited strong concentration-dependent ± antioxidant abilities in vitro. -

Temporal Variation in Community Composition of Root Associated Endophytic Fungi and Carbon and Nitrogen Stable Isotope Abundance in Two Bletilla Species (Orchidaceae)
plants Article Temporal Variation in Community Composition of Root Associated Endophytic Fungi and Carbon and Nitrogen Stable Isotope Abundance in Two Bletilla Species (Orchidaceae) Xinhua Zeng 1, Haixin Diao 1, Ziyi Ni 1, Li Shao 1, Kai Jiang 1 , Chao Hu 1, Qingjun Huang 2 and Weichang Huang 1,3,* 1 Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences, Chenshan Botanical Garden, Shanghai 201620, China; [email protected] (X.Z.); [email protected] (H.D.); [email protected] (Z.N.); [email protected] (L.S.); [email protected] (K.J.); [email protected] (C.H.) 2 Shanghai Institute of Technology, Shanghai 201418, China; [email protected] 3 College of Landscape Architecture, Fujian Agriculture and Forestry University, Fuzhou 350002, China * Correspondence: [email protected] Abstract: Mycorrhizae are an important energy source for orchids that may replace or supplement photosynthesis. Most mature orchids rely on mycorrhizae throughout their life cycles. However, little is known about temporal variation in root endophytic fungal diversity and their trophic functions throughout whole growth periods of the orchids. In this study, the community composition of root endophytic fungi and trophic relationships between root endophytic fungi and orchids were investigated in Bletilla striata and B. ochracea at different phenological stages using stable isotope natural abundance analysis combined with molecular identification analysis. We identified 467 OTUs assigned to root-associated fungal endophytes, which belonged to 25 orders in 10 phyla. Most of these OTUs were assigned to saprotroph (143 OTUs), pathotroph-saprotroph (63 OTUs) and pathotroph- saprotroph-symbiotroph (18 OTUs) using FunGuild database. Among these OTUs, about 54 OTUs Citation: Zeng, X.; Diao, H.; Ni, Z.; could be considered as putative species of orchid mycorrhizal fungi (OMF).