Unique Morphology of the Human Eye and Its Adaptive Meaning
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
pharmaceutics Article Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors Hyeong Min Kim 1,†, Hyounkoo Han 2,†, Hye Kyoung Hong 1, Ji Hyun Park 1, Kyu Hyung Park 1, Hyuncheol Kim 2,* and Se Joon Woo 1,* 1 Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 13620, Korea; [email protected] (H.M.K.); [email protected] (H.K.H.); [email protected] (J.H.P.); [email protected] (K.H.P.) 2 Department of Chemical and Biomolecular Engineering, Sogang University, Seoul 04107, Korea; [email protected] * Correspondence: [email protected] (H.K.); [email protected] (S.J.W.); Tel.: +82-2-705-8922 (H.K.); +82-31-787-7377 (S.J.W.); Fax: +82-2-3273-0331 (H.K.); +82-31-787-4057 (S.J.W.) † These authors contributed equally to this work. Abstract: In this study, Retina-RPE-Choroid-Sclera (RCS) and RPE-Choroid-Sclera (CS) were prepared by scraping them off neural retina, and using the Ussing chamber we measured the average time– concentration values in the acceptor chamber across five isolated rabbit tissues for each drug molecule. We determined the outward direction permeability of the RCS and CS and calculated the neural retina permeability. The permeability coefficients of RCS and CS were as follows: ganciclovir, 13.78 ± 5.82 and 23.22 ± 9.74; brimonidine, 15.34 ± 7.64 and 31.56 ± 12.46; bevacizumab, 0.0136 ± 0.0059 and 0.0612 ± 0.0264 (×10−6 cm/s). -

The Sclera C
The Sclera c. Stephen Foster Maite Sainz de la Maza The Sclera Foreword by Frederick A. lakobiec With 134 Illustrations and 33 Color Plates Springer Science+Business Media, LLC C. Stephen Foster, MD Associate Professor of Ophthalmology Harvard Medical School Director, Immunology and Uveitis Service Massachusetts Eye and Ear Infirmary Boston, MA 02114 USA Maite Sainz de la Maza, MD, PhD Assistant Professor of Ophthalmology Central University of Barcelona 08036 Barcelona Spain Cover illustration: The eye of a patient with rheumatoid arthritis who has developed pro gressively destructive necrotizing scleritis. Library of Congress Cataloging-in-Publication Data Foster, C. Stephen (Charles Stephen), 1942- The sclera/C. Stephen Foster and Maite Sainz de la Maza. p. cm. Includes bibliographical references and index. ISBN 978-1-4757-2345-8 ISBN 978-1-4757-2343-4 (eBook) DOI 10.1007/978-1-4757-2343-4 1. Sclera-Diseases. I. Maza, Maite Sainz de lao II. Title. [DNLM: 1. Scleritis. 2. Sclera. WW 230 F754s 1993] RE328.F67 1993 617.7' 19-dc20 DNLMIDLC for Library of Congress 93-10235 Printed on acid-free paper. © 1994 Springer Science+ Business Media New York Originally published by Springer-Verlag New York, Inc. in 1994 Softcover reprint of the hardcover 1st edition 1994 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher, Springer Science+Business Media, LLC. except for brief excerpts in connection with reviews or, scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. -

Extraocular Muscles Orbital Muscles
EXTRAOCULAR MUSCLES ORBITAL MUSCLES INTRA- EXTRA- OCULAR OCULAR CILIARY MUSCLES INVOLUNTARY VOLUNTARY 1.Superior tarsal muscle. 1.Levator Palpebrae Superioris 2.Inferior tarsal muscle 2.Superior rectus 3.Inferior rectus 4.Medial rectus 5.Lateral rectus 6.Superior oblique 7.Inferior oblique LEVATOR PALPEBRAE SUPERIORIOS Origin- Inferior surface of lesser wing of sphenoid. Insertion- Upper lamina (Voluntary) - Anterior surface of superior tarsus & skin of upper eyelid. Middle lamina (Involuntary) - Superior margin of superior tarsus. (Superior Tarsus Muscle / Muller muscle) Lower lamina (Involuntary) - Superior conjunctival fornix Nerve Supply :- Voluntary part – Oculomotor Nerve Involuntary part – Sympathetic ACTION :- Elevation of upper eye lid C/S :- Drooping of upper eyelid. Congenital ptosis due to localized myogenic dysgenesis Complete ptosis - Injury to occulomotor nerve. Partial ptosis - disruption of postganglionic sympathetic fibres from superior cervical sympathetic ganglion. Extra ocular Muscles : Origin Levator palpebrae superioris Superior Oblique Superior Rectus Lateral Rectus Medial Rectus Inferior Oblique Inferior Rectus RECTUS MUSCLES : ORIGIN • Arises from a common tendinous ring knows as ANNULUS OF ZINN • Common ring of connective tissue • Anterior to optic foramen • Forms a muscle cone Clinical Significance Retrobulbar neuritis ○ Origin of SUPERIOR AND MEDIAL RECTUS are closely attached to the dural sheath of the optic nerve, which leads to pain during upward & inward movements of the globe. Thyroid orbitopathy ○ Medial & Inf.rectus thicken. especially near the orbital apex - compression of the optic nerve as it enters the optic canal adjacent to the body of the sphenoid bone. Ophthalmoplegia ○ Proptosis occur due to muscle laxity. Medial Rectus Superior Rectus Origin :- Superior limb of the tendonous ring, and optic nerve sheath. -

Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception
The Journal of Neuroscience, January 28, 2015 • 35(4):1493–1504 • 1493 Behavioral/Cognitive Action and Perception Are Temporally Coupled by a Common Mechanism That Leads to a Timing Misperception Elena Pretegiani,1,2 Corina Astefanoaei,3 XPierre M. Daye,1,4 Edmond J. FitzGibbon,1 Dorina-Emilia Creanga,3 Alessandra Rufa,2 and XLance M. Optican1 1Laboratory of Sensorimotor Research, NEI, NIH, DHHS, Bethesda, Maryland, 20892-4435, 2EVA-Laboratory, University of Siena, 53100 Siena, Italy, 3Alexandru Ioan Cuza University, Physics Faculty, 700506 Iasi, Romania, and 4Institut du cerveau et de la moelle´pinie e `re (ICM), INSERM UMRS 975, 75013 Paris, France We move our eyes to explore the world, but visual areas determining where to look next (action) are different from those determining what we are seeing (perception). Whether, or how, action and perception are temporally coordinated is not known. The preparation time course of an action (e.g., a saccade) has been widely studied with the gap/overlap paradigm with temporal asynchronies (TA) between peripheral target onset and fixation point offset (gap, synchronous, or overlap). However, whether the subjects perceive the gap or overlap, and when they perceive it, has not been studied. We adapted the gap/overlap paradigm to study the temporal coupling of action and perception. Human subjects made saccades to targets with different TAs with respect to fixation point offset and reported whether they perceived the stimuli as separated by a gap or overlapped in time. Both saccadic and perceptual report reaction times changed in the same way as a function of TA. The TA dependencies of the time change for action and perception were very similar, suggesting a common neural substrate. -

The Complexity and Origins of the Human Eye: a Brief Study on the Anatomy, Physiology, and Origin of the Eye
Running Head: THE COMPLEX HUMAN EYE 1 The Complexity and Origins of the Human Eye: A Brief Study on the Anatomy, Physiology, and Origin of the Eye Evan Sebastian A Senior Thesis submitted in partial fulfillment of the requirements for graduation in the Honors Program Liberty University Spring 2010 THE COMPLEX HUMAN EYE 2 Acceptance of Senior Honors Thesis This Senior Honors Thesis is accepted in partial fulfillment of the requirements for graduation from the Honors Program of Liberty University. ______________________________ David A. Titcomb, PT, DPT Thesis Chair ______________________________ David DeWitt, Ph.D. Committee Member ______________________________ Garth McGibbon, M.S. Committee Member ______________________________ Marilyn Gadomski, Ph.D. Assistant Honors Director ______________________________ Date THE COMPLEX HUMAN EYE 3 Abstract The human eye has been the cause of much controversy in regards to its complexity and how the human eye came to be. Through following and discussing the anatomical and physiological functions of the eye, a better understanding of the argument of origins can be seen. The anatomy of the human eye and its many functions are clearly seen, through its complexity. When observing the intricacy of vision and all of the different aspects and connections, it does seem that the human eye is a miracle, no matter its origins. Major biological functions and processes occurring in the retina show the intensity of the eye’s intricacy. After viewing the eye and reviewing its anatomical and physiological domain, arguments regarding its origins are more clearly seen and understood. Evolutionary theory, in terms of Darwin’s thoughts, theorized fossilization of animals, computer simulations of eye evolution, and new research on supposed prior genes occurring in lower life forms leading to human life. -
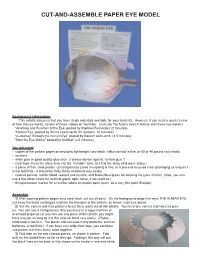
Cut-And-Assemble Paper Eye Model
CUT-AND-ASSEMBLE PAPER EYE MODEL Background information: This activity assumes that you have study materials available for your students. However, if you need a quick review of how the eye works, try one of these videos on YouTube. (Just use YouTube’s search feature with these key words.) “Anatomy and Function of the Eye: posted by Raphael Fernandez (2 minutes) “Human Eye” posted by Smart Learning for All (cartoon, 10 minutes) “A Journey Through the Human Eye” posted by Bausch and Lomb (2.5 minutes) “How the Eye Works” posted by AniMed (2.5 minutes) You will need: • copies of the pattern pages printed onto lightweight card stock (vellum bristol is fine, or 65 or 90 pound card stock) • scissors • white glue or good quality glue stick (I always advise against “school glue.”) • clear tape (I use the shiny kind, not the “invisible” kind, as I find the shiny kind more sticky.) • a piece of thin, clear plastic (a transparency [used in copiers] is fine, or a piece of recycled clear packaging as long as it is not too thick-- it should be fairly flimsy and bend very easily) • colored pencils: red for blood vessels and muscle, and brown/blue/green for coloring iris (your choice) (Also, you can use a few other colors for lacrimal gland, optic nerve, if you want to.) • thin permanent marker for a number labels on plastic parts (such as a very thin point Sharpie) Assembly: 1) After copying pattern pages onto card stock, cut out all parts. On the background page that says THE HUMAN EYE, cut away the black rectangles and trim the triangles at the bottom, as shown in picture above. -

The Proteomes of the Human Eye, a Highly Compartmentalized Organ
Proteomics 17, 1–2, 2017, 1600340 DOI 10.1002/pmic.201600340 (1 of 3) 1600340 The proteomes of the human eye, a highly compartmentalized organ Gilbert S. Omenn Center for Computational Medicine and Bioinformatics, University of Michigan, Ann Arbor, MI, USA Proteomics has now published a series of Dataset Briefs on the EyeOme from the HUPO Received: November 2, 2016 Human Proteome Project with high-quality analyses of the proteomes of these compartments Accepted: November 4, 2016 of the human eye: retina, iris, ciliary body, retinal pigment epithelium/choroid, retrobulbar optic nerve, and sclera, with 3436, 2929, 2867, 2755, 2711, and 1945 proteins, respectively. These proteomics resources represent a useful starting point for a broad range of research aimed at developing preventive and therapeutic interventions for the various causes of blindness. Keywords: Biomedicine / Biology and Disease-driven Human Proteome Project / End Blindness by 2020 / Eye proteome / EyeOme / Human Proteome Project See accompanying articles in the EyeOme series: http://dx.doi.org/10.1002/pmic.201600229; http://dx.doi.org/10.1002/pmic.201500188; http://dx.doi.org/10.1002/pmic.201400397 Proteomics has now published a series of four papers on compartments of the eye as shown in Fig. 1. As was noted [5], the human eye proteome [1–4]. Under the aegis of the Hu- it was not feasible to assess the quality of the data or estimate man Proteome Organization Biology and Disease-driven Hu- numbers of likely false positives in the heterogeneous studies man Proteome Project (HPP), the EyeOme was organized by from which these findings were summarized. -

The Evolution of Human Intelligence and the Coefficient of Additive Genetic Variance in Human Brain Size ⁎ Geoffrey F
Intelligence 35 (2007) 97–114 The evolution of human intelligence and the coefficient of additive genetic variance in human brain size ⁎ Geoffrey F. Miller a, , Lars Penke b a University of New Mexico, USA b Institut für Psychologie, Humboldt-Universität zu Berlin, Germany Received 3 November 2005; received in revised form 17 August 2006; accepted 18 August 2006 Available online 12 October 2006 Abstract Most theories of human mental evolution assume that selection favored higher intelligence and larger brains, which should have reduced genetic variance in both. However, adult human intelligence remains highly heritable, and is genetically correlated with brain size. This conflict might be resolved by estimating the coefficient of additive genetic variance (CVA) in human brain size, since CVAs are widely used in evolutionary genetics as indexes of recent selection. Here we calculate for the first time that this CVA is about 7.8, based on data from 19 recent MRI studies of adult human brain size in vivo: 11 studies on brain size means and standard deviations, and 8 studies on brain size heritabilities. This CVA appears lower than that for any other human organ volume or life-history trait, suggesting that the brain has been under strong stabilizing (average-is-better) selection. This result is hard to reconcile with most current theories of human mental evolution, which emphasize directional (more-is-better) selection for higher intelligence and larger brains. Either these theories are all wrong, or CVAs are not as evolutionarily informative as most evolutionary geneticists believe, or, as we suggest, brain size is not a very good index for understanding the evolutionary genetics of human intelligence. -

Scleral Lenses and Eye Health
Scleral Lenses and Eye Health Anatomy and Function of the Human Eye How Scleral Lenses Interact with the Ocular Surface Just as the skin protects the human body, the ocular surface protects the human Scleral lenses are large-diameter lenses designed to vault the cornea and rest on the conjunctival tissue sitting on eye. The ocular surface is made up of the cornea, the conjunctiva, the tear film, top of the sclera. The space between the back surface of the lens and the cornea acts as a fluid reservoir. Scleral and the glands that produce tears, oils, and mucus in the tear film. lenses can range in size from 13mm to 19mm, although larger diameter lenses may be designed for patients with more severe eye conditions. Due to their size, scleral lenses consist SCLERA: The sclera is the white outer wall of the eye. It is SCLERAL LENS made of collagen fibers that are arranged for strength rather of at least two zones: than transmission of light. OPTIC ZONE The optic zone vaults over the cornea CORNEA: The cornea is the front center portion of the outer Cross section of FLUID RESERVOIR wall of the eye. It is made of collagen fibers that are arranged in the eye shows The haptic zone rests on the conjunctiva such a way so that the cornea is clear. The cornea bends light the cornea, overlying the sclera as it enters the eye so that the light is focused on the retina. conjunctiva, and sclera as CORNEA The cornea has a protective surface layer called the epithelium. -

Arteriovenous Dissection in a Living Human
Vienna, Austria, 1990. Dordrecht, Holland: Klu- Table 2. Ultrasonographic Findings of 25 Well-Documented Patients wer Academic Publishers; 1993:307-311. With Cavitary Melanoma of the Uvea in English Literature 9. Frazier-Byrne S, Green RL. Intraocular tumors. In: Frazier-Byrne S, Green RL, eds. Ultrasound of the Eye and Orbit. 2nd ed. St Louis, Mo: Ultrasonographic Findings Mosby; 2002:115-190. 10. Scott CT, Holland GN, Glasgow BJ. Cavita- Solid % Mass tion in ciliary body melanoma. Am J Ophthalmol. Component Loculation Echoes in Septa in Thickness Occupied 1997;123:269-271. Source Present on USG Cavitation Cavitation by Cavity 11. Cohen PR, Rapini RP. Nevus with cyst: a re- port of 93 cases. Am J Dermatopathol. 1993; Kennedy5 NA NA NA NA NA 15:229-234. NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA Reese6 NA NA NA NA NA Arteriovenous Dissection Zakka et al7 ϩ Unilocular ϩ −NA in a Living Human Eye: Stone and Shapiro4 ϩ Unilocular ϩ −65 ϩ Unilocular ϩ −75 Clinicopathologic − Unilocular ϩ −60 Correlation ϩ Unilocular − − 30 ϩ Multilocular ϩϩ 40 Fledelius et al8 − Unilocular − − 75 Although the visual results after ar- Scott et al10 − Multilocular ϩϩ NA teriovenous dissection (AVD) seem 1,2 Lois et al2 − Unilocular ϩ −79 encouraging, its effectiveness has ϩ Unilocular − − 59 not been proved in a controlled, pro- ϩ Multilocular − ϩ 31 spective clinical trial. The role of sur- ϩ Multilocular ϩϩ 59 gical decompression itself remains ϩ − Unilocular −64 unclear,3 and little is known about − Multilocular ϩϩ 62 ϩ Unilocular ϩ −55 surgically induced nerve fiber de- ϩ Multilocular ϩϩ 38 fects. -

Root Eye Dictionary a "Layman's Explanation" of the Eye and Common Eye Problems
Welcome! This is the free PDF version of this book. Feel free to share and e-mail it to your friends. If you find this book useful, please support this project by buying the printed version at Amazon.com. Here is the link: http://www.rooteyedictionary.com/printversion Timothy Root, M.D. Root Eye Dictionary A "Layman's Explanation" of the eye and common eye problems Written and Illustrated by Timothy Root, M.D. www.RootEyeDictionary.com 1 Contents: Introduction The Dictionary, A-Z Extra Stuff - Abbreviations - Other Books by Dr. Root 2 Intro 3 INTRODUCTION Greetings and welcome to the Root Eye Dictionary. Inside these pages you will find an alphabetical listing of common eye diseases and visual problems I treat on a day-to-day basis. Ophthalmology is a field riddled with confusing concepts and nomenclature, so I figured a layman's dictionary might help you "decode" the medical jargon. Hopefully, this explanatory approach helps remove some of the mystery behind eye disease. With this book, you should be able to: 1. Look up any eye "diagnosis" you or your family has been given 2. Know why you are getting eye "tests" 3. Look up the ingredients of your eye drops. As you read any particular topic, you will see that some words are underlined. An underlined word means that I've written another entry for that particular topic. You can flip to that section if you'd like further explanation, though I've attempted to make each entry understandable on its own merit. I'm hoping this approach allows you to learn more about the eye without getting bogged down with minutia .. -

98796-Anatomy of the Orbit
Anatomy of the orbit Prof. Pia C Sundgren MD, PhD Department of Diagnostic Radiology, Clinical Sciences, Lund University, Sweden Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lay-out • brief overview of the basic anatomy of the orbit and its structures • the orbit is a complicated structure due to its embryological composition • high number of entities, and diseases due to its composition of ectoderm, surface ectoderm and mesoderm Recommend you to read for more details Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 3 x 3 Imaging technique 3 layers: - neuroectoderm (retina, iris, optic nerve) - surface ectoderm (lens) • CT and / or MR - mesoderm (vascular structures, sclera, choroid) •IOM plane 3 spaces: - pre-septal •thin slices extraconal - post-septal • axial and coronal projections intraconal • CT: soft tissue and bone windows 3 motor nerves: - occulomotor (III) • MR: T1 pre and post, T2, STIR, fat suppression, DWI (?) - trochlear (IV) - abducens (VI) Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Superior orbital fissure • cranial nerves (CN) III, IV, and VI • lacrimal nerve • frontal nerve • nasociliary nerve • orbital branch of middle meningeal artery • recurrent branch of lacrimal artery • superior orbital vein • superior ophthalmic vein Lund University / Faculty of Medicine / Inst. Clinical Sciences / Radiology / ECNR Dubrovnik / Oct 2018 Lund University / Faculty of Medicine / Inst.