Ust Be Reregistration Registered by EPA, Based on Scientific Studies Showing That They Can Be Used Without Posing Unreasonable Risks to People Or the Environment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Validation of a Method for the Simultaneous
CORE Metadata, citation and similar papers at core.ac.uk Provided by UGD Academic Repository American Journal of Applie d Chemistry 2014; 2(4): 46-54 Published online August 10, 2014 (http://www.sciencepublishinggroup.com/j/ajac) doi: 10.11648/j.ajac.20140204.11 ISSN: 2330-8753 (Print); ISSN: 2330-8745 (Online) Development and validation of a method for the simultaneous determination of 20 organophosphorus pesticide residues in corn by accelerated solvent extraction and gas chromatography with nitrogen phosphorus detection Vesna Kostik *, Biljana Gjorgeska, Bistra Angelovska Medical Faculty, Department of Pharmacy, University “Goce Delchev”, Shtip, Republic of Macedonia Email address: [email protected] (V. Kostik), [email protected] (B. Gjorgeska), [email protected] (B. Angelovska) To cite this article: Vesna Kostik, Biljana Gjorgeska, Bistra Angelovska. Development and Validation of a Method for the Simultaneous Determination of 20 Organophosphorus Pesticide Residues in Corn by Accelerated Solvent Extraction and Gas Chromatography with Nitrogen Phosphorus Detection. American Journal of Applied Chemistry. Vol. 2, No. 4, 2014, pp. 46-54. doi: 10.11648/j.ajac.20140204.11 Abstract: The method for simultaneous determination of 20 organophosphorus pesticide residues in corn samples has been developed and validated. For the extraction of organophosporus pesticide residues from the samples, the accelerated solvent technique with the mixture of dichloromethane: acetone (1:1, V/V ) was used. Clean up was done using liquid – liquid extraction with n – hexane, followed by solid phase extraction on primary secondary amine adsorbent, and elution with the mixture of acetone: toluene (65:35). The determination of the pesticides was carried out by gas chromatography with nitrogen phosphorus detection. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

Table II. EPCRA Section 313 Chemical List for Reporting Year 2017 (Including Toxic Chemical Categories)
Table II. EPCRA Section 313 Chemical List For Reporting Year 2017 (including Toxic Chemical Categories) Individually listed EPCRA Section 313 chemicals with CAS numbers are arranged alphabetically starting on page II-3. Following the alphabetical list, the EPCRA Section 313 chemicals are arranged in CAS number order. Covered chemical categories follow. Note: Chemicals may be added to or deleted from the list. The Emergency Planning and Community Right-to-Know Call Center or the TRI-Listed Chemicals website will provide up-to-date information on the status of these changes. See section B.3.c of the instructions for more information on the de minimis % limits listed below. There are no de minimis levels for PBT chemicals since the de minimis exemption is not available for these chemicals (an asterisk appears where a de minimis limit would otherwise appear in Table II). However, for purposes of the supplier notification requirement only, such limits are provided in Appendix C. Chemical Qualifiers Certain EPCRA Section 313 chemicals listed in Table II have parenthetic “qualifiers.” These qualifiers indicate that these EPCRA Section 313 chemicals are subject to the section 313 reporting requirements if manufactured, processed, or otherwise used in a specific form or when a certain activity is performed. An EPCRA Section 313 chemical that is listed without a qualifier is subject to reporting in all forms in which it is manufactured, processed, and otherwise used. The following chemicals are reportable only if they are manufactured, processed, or otherwise used in the specific form(s) listed below: Chemical/ Chemical Category CAS Number Qualifier Aluminum (fume or dust) 7429-90-5 Only if it is a fume or dust form. -

Amending Annex II to Directives 76/895/EEC and 86/362/EEC
20 . 5 . 88 Official Journal of the European Communities No L 126/53 COUNCIL DIRECTIVE of 16 May 1988 amending Annex II to Directives 76/895/EEC and 86/362/EEC relating to the fixing of maximum levels for pesticide residues in and on fruit and vegetables and cereals respectively (88/298/EEC) THE COUNCIL OF THE EUROPEAN COMMUNITIES, Having regard to the Treaty establishing the European Economic Community, Having regard to Council Directive 76/895/EEC of 23 November 1976 relating to the fixing of maximum levels for pesticide residues in and on fruit and vegetables 0), as last amended by Regulation (EEC) No 3768/85 (2), and in particular Article 5 thereof, Having regard to Council Directive 86/362/EEC of 24 July 1986 on the fixing of maximum levels for pesticide residues in and on cereals (3), and in particular Article 1 1 thereof, Having regard to the proposal from the Commission, Whereas, in the light of technical and scientific progress and of the requirements of public health and agriculture, it is necessary to amend the provisions, particularly the maximum levels, contained in Annex II to Directive 76/895/EEC relating to captafol, captan, chlorfenvinphos, dodine, fenitrothion, folpet, formothion and malathion ; Whereas, for the same reasons, it seems desirable to update Directive 76/895/EEC by adding provisions relating to further pesticides, residues of which may occur in and on fruit and vegetables, namely, ethion, ethylene dibromide, mevinphos, phosalone and 2, 4, 5-T, arid to update Directive 86/362/EEC by adding provisions relating to a further pesticide the residues of which may occur in cereals, namely, captafol, HAS ADOPTED THIS DIRECTIVE : Article 1 Annex II to Directive 76/895/EEC is hereby amended as follows : 1 . -

SECRETARY HICKEL BANS USE of 16 PESTICIDES on ANY INTERIOR LANDS OR PROGRAMS -- June 18, 1970
i DEPARTMENT of the INTERIOR news release OFFICE OF THE SECRETARY For Release Thursday, June 18, 1970 SECRETARYHICKEL BANS USE OF 16 PESTICIDES ON ANY INTERIOR LANDS OR PROGRAMS Secretary of the Interior Walter J. Hickel today announced a new policy flatly banning the use of 16 types of pesticides on any lands managed by the Department's bureaus and agencies, or in any program run by them. The Department administers approximately 70 percent of all federally owned lands. Included in this list of prohibited pesticides are such widely-known substances as DDT, Aldrin, 2, 4, 5,-T, Dieldrin, Endrin, Heptachlor, Lindane and Toxaphene. Also on the list of unconditionally banned items are Amitrol, arsenical compounds (inorganic), Azodrin, Bidrin, DDD (TDE), mercurial compounds, Strobane, and Thallium Sulfate. The Secretary pointed out that nearly all use of these pesticides has been banned on Interior Lands during recent years. The purpose of the new statement, he said, is to establish a policy for guidance of all Interior personnel. Another group of chemical pesticides, titled the Restricted List, are to be used only when non-chemical techniques have been considered and found inadequate, and when use can be limited to small-scale applications. Secretary Hickel emphasized that the use of any chemical pesticide by his agencies must be aimed at a specific pest problem, and involve minimum strength and minimum frequency of application. "We in the Interior Department-- the Nation's chief conservation agency have a special obligation to protect the environment for all the people,“’ Secretary Hickel said. "We are charged by law with responsibility for protecting interstate and coastal water quality, our fish and wildlife resources, the integrity of our national parks, public lands, and recreation areas," the Secretary continued. -

Acutely / Extremely Hazardous Waste List
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extemely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extemely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extemely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extemely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extemely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extemely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extemely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extemely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extemely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extemely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extemely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Quantification of Organophosphorus Pesticides by Quechers and LC
HPLC Application ID No.: 20020 Quantification of Organophosphorus Pesticides by QuEchERS and LC/MS/MS Column: Synergi™ 4 µm Fusion-RP 80 Å, LC Column 50 x 2 mm, Ea Dimensions: 50 x 2 mm ID Order No: 00B-4424-B0 Elution Type: Gradient Eluent A: Add 5mL of 1M ammonium formate to 1L Water Eluent B: Add 5mL of 1M ammonium formate to 1L Methanol Gradient Step No. Time (min) Pct A Pct B Profile: 1 -5 95 5 2 0 95 5 3 2 95 5 4 6 50 50 5 12 10 90 6 14 10 90 7 16 95 5 8 17 95 5 Flow Rate: 250 mL/min Col. Temp.: 25 °C Detection: Tandem Mass Spec (MS-MS) @ (ambient) Detector Info: <a target="_blank" href="https://sciex.com/products/mass-spectrometers?utm_campaign=2019%20application%20search&utm_source=phenomenex&utm_medium=referral">SCIEX</a> Analyst Note: SecurityGuard™ Guard Cartridge System extends column lifetime. - SecurityGuard Cartridges, Fusion-RP 4 x 2.0mm ID, 10/Pk Part No.: AJ0-7556 - Holder Part No.: KJ0-4282 ©2021 Phenomenex Inc. All rights reserved. For more information contact your Phenomenex Representative at [email protected] Phenomenex products are available worldwide. www.phenomenex.com [email protected] Page 1 of 2 HPLC Application ID No.: 20020 Quantification of Organophosphorus Pesticides by QuEchERS and LC/MS/MS ANALYTES: 1 Acephate (MRM: 184/142.8) 41 Diazinon (MRM: 305/169) 2 Acephate (MRM: 184/94.9) 42 Diazinon (MRM: 305/153) 3 Demeton-S-methyl sulfone (MRM: 263/169) 43 Fenthion (MRM: 278.9/169) 4 Demeton-S-methyl sulfone (MRM: 263/109) 44 Fenthion (MRM: 278.9/246.9) 5 Parathion-methyl Methyl parathion (MRM: 263.9/232.1)45 -
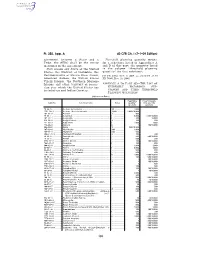
40 CFR Ch. I (7–1–09 Edition) Pt. 355, App. A
Pt. 355, App. A 40 CFR Ch. I (7–1–09 Edition) agreement between a State and a Threshold planning quantity means, Tribe, the SERC shall be the entity for a substance listed in Appendices A identified in the agreement. and B of this part, the quantity listed State means any State of the United in the column ‘‘threshold planning States, the District of Columbia, the quantity’’ for that substance. Commonwealth of Puerto Rico, Guam, [73 FR 65462, Nov. 3, 2008, as amended at 73 American Samoa, the United States FR 76960, Dec. 18, 2008] Virgin Islands, the Northern Mariana Islands, any other territory or posses- APPENDIX A TO PART 355—THE LIST OF sion over which the United States has EXTREMELY HAZARDOUS SUB- jurisdiction and Indian Country. STANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. 10 ................ 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... 1,000 ........... 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... 1 .................. 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f ................... 100 10,000 -

Insecticides
fY)I\) -;; ooo 3tfJ INSECTICIDES Extension Bulletin 387-Revlsed 1980 AGAfCULTURAL EXTENSION SERVICE UNIVERSITY OF MINNESOTA Contents General precautions for using pesticides . 4 Safety precautions and first aid . 4 Minnesota poison information centers . 5 Protecting honey bees from insecticides . 6 Pesticide toxicity and LD 50's . • . • . 6 Acute oral and dermal LD 50's for insecticides . 7 Forms of insecticides . 8 Calculating dosage and rates of application . 9 Sprayer calibration . 11 Description of insecticides, miticides ........................... 12 Chlorinated hydrocarbons ................................. 12 Carbamates .............................................. 13 Organophosphates ........................................ 14 Sulfonates, carbonates, botanicals, and miscellaneous groups ... 18 Legal Restrictions on Use of Pesticides The Federal Insecticide, Fungicide and Rodenticide Act and the Minnesota Pesticide Act of 1976, require that those who use or supervise the use of certain pesticides with restricted uses must be certified. The labels of those pesticides with restricted uses will contain information regarding these restrictions. Be sure to read all labels thoroughly and use any pesticide for the crops and pests listed on the label only. Information about applicator certification may be obtained from your County Extension Director or the Minnesota Depmt ment of Agriculture. The U.S. Environmental Protection Agency (EPA) has designated the following pesticides for reshicted use: acrolein endrin mevinphos (Phosdrin) acrylonitrile ethyl parathion paraquat aldicarb (Temik) 1080 piclorarri (Tordon) allyl alcohol 1081 sodium cyanide alluminum phosphide (Phostoxin) hydrocyanic acid strychnine azinphos methyl (Guthion) methomyl (Lannate, Nudrin) sulfotepp calcium cyanide methyl bromide tepp demeton (Systox) methyl parathion Authors of this publication are J. A. Lofgren, professor and extension entomologist; D. M. Noetzel, assistant professor and extension entomologist; P. K. Hareln, professor and extension entomologist; M. -

Environmental Protection Agency Pt. 355, App. A
Environmental Protection Agency Pt. 355, App. A agreement between a State and a sion over which the United States has Tribe, the SERC shall be the entity jurisdiction and Indian Country. identified in the agreement. Threshold planning quantity means, State means any State of the United for a substance listed in Appendices A States, the District of Columbia, the and B of this part, the quantity listed Commonwealth of Puerto Rico, Guam, in the column ‘‘threshold planning American Samoa, the United States quantity’’ for that substance. Virgin Islands, the Northern Mariana [73 FR 65462, Nov. 3, 2008, as amended at 73 Islands, any other territory or posses- FR 76960, Dec. 18, 2008] APPENDIX A TO PART 355—THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. ..................... 10 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... ..................... 1,000 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... ..................... 1 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f .................. -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation.