View Full Text Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chinese Rhubarb)
IJAS_39192 Vol 8, Issue 6, 2020 ISSN- 2321-6832 Review Article GENERAL OVERVIEW OF PHYTOCHEMISTRY AND PHARMACOLOGICAL POTENTIAL OF RHEUM PALMATUM (CHINESE RHUBARB) AAMIR KHAN KHATTAK, SYEDA MONA HASSAN, SHAHZAD SHARIF MUGHAL* Department of Chemistry, Lahore Garrison University, Lahore, Pakistan, Email: [email protected] Received: 21 July 2020 , Revised and Accepted: 11 October 2020 ABSTRACT Recent probe of medicinal plants incorporated in traditional systems for curing infection and sustaining holistic health, has exposed good sum of therapeutic efficiency against deleterious infections and chronic illnesses. Rheum palmatum (Chinese Rhubarb, family Polygonaceae) is a significant medicinal herb, which finds an extensive use in Unani (Traditional) system of medicine. It has been traditionally employed as antiseptic, liver stimulant, diuretic, diabetes, stomachic, purgative/cathartic, anticholesterolemic, antitumor, Alzheimer’s, Parkinson’s, tonic, antidiabetic, and wound healer. The most vital components from Rheum palmatum are the phenolics, flavonoids, terpenoids, saponins, and anthraquinone derivatives such as aloe- emodin, chrysophanol, physcion, rhein, emodin and its glucorhein, and glycoside. Rhubarb also contains tannins which include hydrolysable-tannins, containing glycosidic or ester bonds composed of glucose, gallic acid, and other monosaccharide’s and condensed tannins, resulting principally from the flavone derivatives leukocyanidin and catechin. In recent years, new components such asrevandchinone-1, revandchinone-2, revandchinone-3, revandchinone-4, sulfemodin8-O-b-Dglucoside, and 6-methyl-rhein and aloe-emodin have been reported from the same class. It also encompasses some macro and micro mineral elements such as Ca, K, Mn, Fe, Co, Zn, Na, Cu, and Li. Anthraquinone derivatives demonstrate evidence of anti- microbial, antifungal, anti-proliferative, anti-Parkinson’s, immune enhancing, anticancer, antiulcer, antioxidant, and antiviral activities. -
(A) Journals with the Largest Number of Papers Reporting Estimates Of
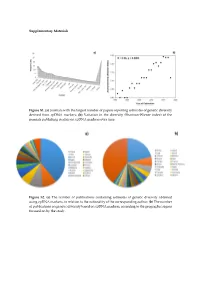
Supplementary Materials Figure S1. (a) Journals with the largest number of papers reporting estimates of genetic diversity derived from cpDNA markers; (b) Variation in the diversity (Shannon-Wiener index) of the journals publishing studies on cpDNA markers over time. Figure S2. (a) The number of publications containing estimates of genetic diversity obtained using cpDNA markers, in relation to the nationality of the corresponding author; (b) The number of publications on genetic diversity based on cpDNA markers, according to the geographic region focused on by the study. Figure S3. Classification of the angiosperm species investigated in the papers that analyzed genetic diversity using cpDNA markers: (a) Life mode; (b) Habitat specialization; (c) Geographic distribution; (d) Reproductive cycle; (e) Type of flower, and (f) Type of pollinator. Table S1. Plant species identified in the publications containing estimates of genetic diversity obtained from the use of cpDNA sequences as molecular markers. Group Family Species Algae Gigartinaceae Mazzaella laminarioides Angiospermae Typhaceae Typha laxmannii Angiospermae Typhaceae Typha orientalis Angiospermae Typhaceae Typha angustifolia Angiospermae Typhaceae Typha latifolia Angiospermae Araliaceae Eleutherococcus sessiliflowerus Angiospermae Polygonaceae Atraphaxis bracteata Angiospermae Plumbaginaceae Armeria pungens Angiospermae Aristolochiaceae Aristolochia kaempferi Angiospermae Polygonaceae Atraphaxis compacta Angiospermae Apocynaceae Lagochilus macrodontus Angiospermae Polygonaceae Atraphaxis -

Widespread Paleopolyploidy, Gene Tree Conflict, and Recalcitrant Relationships Among the 3 Carnivorous Caryophyllales1 4 5 Joseph F
bioRxiv preprint doi: https://doi.org/10.1101/115741; this version posted March 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 2 Widespread paleopolyploidy, gene tree conflict, and recalcitrant relationships among the 3 carnivorous Caryophyllales1 4 5 Joseph F. Walker*,2, Ya Yang2,5, Michael J. Moore3, Jessica Mikenas3, Alfonso Timoneda4, Samuel F. 6 Brockington4 and Stephen A. Smith*,2 7 8 2Department of Ecology & Evolutionary Biology, University of Michigan, 830 North University Avenue, 9 Ann Arbor, MI 48109-1048, USA 10 3Department of Biology, Oberlin College, Science Center K111, 119 Woodland St., Oberlin, Ohio 44074- 11 1097 USA 12 4Department of Plant Sciences, University of Cambridge, Cambridge CB2 3EA, United Kingdom 13 5 Department of Plant Biology, University of Minnesota-Twin Cities. 1445 Gortner Avenue, St. Paul, MN 14 55108 15 CORRESPONDING AUTHORS: Joseph F. Walker; [email protected] and Stephen A. Smith; 16 [email protected] 17 18 1Manuscript received ____; revision accepted ______. bioRxiv preprint doi: https://doi.org/10.1101/115741; this version posted March 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 19 ABSTRACT 20 • The carnivorous members of the large, hyperdiverse Caryophyllales (e.g. -

UHPLC Analysis of Reynoutria Japonica Houtt. Rhizome Preparations Regarding Stilbene and Anthranoid Composition and Their Antimycobacterial Activity Evaluation
plants Article UHPLC Analysis of Reynoutria japonica Houtt. Rhizome Preparations Regarding Stilbene and Anthranoid Composition and Their Antimycobacterial Activity Evaluation Fabian Alperth, Lena Melinz, Johannes-Paul Fladerer and Franz Bucar * Institute of Pharmaceutical Sciences, University of Graz, Beethovenstraße 8, 8010 Graz, Austria; [email protected] (F.A.); [email protected] (L.M.); johannes.fl[email protected] (J.-P.F.) * Correspondence: [email protected]; Tel.: +43-316-380-5531 Abstract: Reynoutria japonica Houtt. is a critical invasive alien plant in Europe and North America with a drastic impact on native flora. However, R. japonica has medicinal potential, especially as a source of stilbenes. In order to explore the potential of simple extractions of R. japonica, we conducted qualitative and quantitative analyses of fresh R. japonica rhizome infusion, decoction, and macerates with ethanol by UHPLC-DAD-ESI-MSn and UHPLC-DAD, with a focus on major constituent groups of stilbenes and anthranoids. Since R. japonica rhizome extracts showed antimicrobial potential in the past, we also evaluated the antimycobacterial effect of raw R. japonica extracts for the first time against Mycobacterium smegmatis. Of thirty-four characterized substances, six were stilbenes and twelve anthranoids. The main constituents, four trans-stilbenes and eight anthranoids, were quantified in a validated UHPLC-DAD method. The 38% ethanol macerate showed high stilbene (155.078 mg/100 g Citation: Alperth, F.; Melinz, L.; fluid extract) and low anthranoid content (5.420 mg/100 g fluid extract), while decoction showed the Fladerer, J.-P.; Bucar, F. UHPLC µ Analysis of Reynoutria japonica Houtt. highest anthranoids. -

Full of Beans: a Study on the Alignment of Two Flowering Plants Classification Systems
Full of beans: a study on the alignment of two flowering plants classification systems Yi-Yun Cheng and Bertram Ludäscher School of Information Sciences, University of Illinois at Urbana-Champaign, USA {yiyunyc2,ludaesch}@illinois.edu Abstract. Advancements in technologies such as DNA analysis have given rise to new ways in organizing organisms in biodiversity classification systems. In this paper, we examine the feasibility of aligning two classification systems for flowering plants using a logic-based, Region Connection Calculus (RCC-5) ap- proach. The older “Cronquist system” (1981) classifies plants using their mor- phological features, while the more recent Angiosperm Phylogeny Group IV (APG IV) (2016) system classifies based on many new methods including ge- nome-level analysis. In our approach, we align pairwise concepts X and Y from two taxonomies using five basic set relations: congruence (X=Y), inclusion (X>Y), inverse inclusion (X<Y), overlap (X><Y), and disjointness (X!Y). With some of the RCC-5 relationships among the Fabaceae family (beans family) and the Sapindaceae family (maple family) uncertain, we anticipate that the merging of the two classification systems will lead to numerous merged solutions, so- called possible worlds. Our research demonstrates how logic-based alignment with ambiguities can lead to multiple merged solutions, which would not have been feasible when aligning taxonomies, classifications, or other knowledge or- ganization systems (KOS) manually. We believe that this work can introduce a novel approach for aligning KOS, where merged possible worlds can serve as a minimum viable product for engaging domain experts in the loop. Keywords: taxonomy alignment, KOS alignment, interoperability 1 Introduction With the advent of large-scale technologies and datasets, it has become increasingly difficult to organize information using a stable unitary classification scheme over time. -

-

Morphological Traits of Gynodioecious Persicaria Amphibia (Polygonaceae)
Phytotaxa 219 (2): 133–143 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2015 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.219.2.3 Morphological traits of gynodioecious Persicaria amphibia (Polygonaceae) HYE-KYOUNG MOON1 & SUK-PYO HONG1* Laboratory of Plant Systematics, Department of Biology and Research Institute for Basic Sciences, Kyung Hee University, Seoul 130- 701, South Korea; e-mails: [email protected], [email protected] *Author for correspondence Abstract Gynodioecy, as intermediate sexual system, involves modifications of floral structure from hermaphrodites to dioecy. We here present the morphological differences in the sexual type of flowers concerning the terrestrial Persicaria amphibia as a gynodioecious species. Hermaphroditic flowers are relatively larger than female ones, and they produce viable pollen grains, while female flowers produce no viable pollen as male-sterility but increase female fitness by exerted pistil. Although female flowers had aborted stamens, the anther development at the early stage was normal until the late stage which the anther locule of female flowers was collapsed without forming pollen-like structures. The pilate-glandular trichomes could be diagnostic characteristic of female individuals since this type of trichome occurs only in leaves, stems, and pedicels of female plants. Excepting for the trichome type, the variance of microstructures was not significant between hermaphroditic and female plants. Keywords: floral dimorphism, Persicaria amphibia, Polygonaceae, sexual system Introduction Flowering plants are predominantly hermaphroditic, containing both androecium and gynoecium for each flower (see e.g., Barrett 2002). Although the hermaphrodite is a successful reproduction system, opportunities for self-fertilization exist. -

Polygonaceae of Alberta
AN ILLUSTRATED KEY TO THE POLYGONACEAE OF ALBERTA Compiled and writen by Lorna Allen & Linda Kershaw April 2019 © Linda J. Kershaw & Lorna Allen This key was compiled using informaton primarily from Moss (1983), Douglas et. al. (1999) and the Flora North America Associaton (2005). Taxonomy follows VAS- CAN (Brouillet, 2015). The main references are listed at the end of the key. Please let us know if there are ways in which the kay can be improved. The 2015 S-ranks of rare species (S1; S1S2; S2; S2S3; SU, according to ACIMS, 2015) are noted in superscript (S1;S2;SU) afer the species names. For more details go to the ACIMS web site. Similarly, exotc species are followed by a superscript X, XX if noxious and XXX if prohibited noxious (X; XX; XXX) according to the Alberta Weed Control Act (2016). POLYGONACEAE Buckwheat Family 1a Key to Genera 01a Dwarf annual plants 1-4(10) cm tall; leaves paired or nearly so; tepals 3(4); stamens (1)3(5) .............Koenigia islandica S2 01b Plants not as above; tepals 4-5; stamens 3-8 ..................................02 02a Plants large, exotic, perennial herbs spreading by creeping rootstocks; fowering stems erect, hollow, 0.5-2(3) m tall; fowers with both ♂ and ♀ parts ............................03 02b Plants smaller, native or exotic, perennial or annual herbs, with or without creeping rootstocks; fowering stems usually <1 m tall; fowers either ♂ or ♀ (unisexual) or with both ♂ and ♀ parts .......................04 3a 03a Flowering stems forming dense colonies and with distinct joints (like bamboo -

Characteristics That Make the Fallopia Genus (Polygonaceae) Highly Invasive
Ecological Questions 16/2012: 23 – 27 DOI: 10.2478/v10090-012-0002-6 Characteristics that make the Fallopia genus (Polygonaceae) highly invasive Justyna Sołtysiak, Teresa Brej Department of Botany and Plant Ecology, Wrocław University of Environmental and Life Sciences, pl. Grunwaldzki 24a, 50–363 Wrocław, Poland e-mail: [email protected] Abstract. Representatives of the Fallopia genus: Fallopia japonica, Fallopia sachalinensis and Fallopia × bohemica are known as successful invaders, wide spread throughout Europe and North America. This paper focuses on the invasive Fallopia complex and presents some features (a wide ecological amplitude, high competition abilities, sexual reproduction by hybridization) responsible for the fact that all species of the Fallopia genus are aggressive and noxious invaders. Key words: plant invasion, Fallopia japonica, Fallopia sachalinensis, Fallopia × bohemica. 1. Introduction cies of exotic plants have been introduced as a crop and have escaped to become established in natural ecosystems Biological invasions belong to the main problems of con- (Pimentel et al. 2007). temporary ecology and they are considered as a signifi- Recent research were dedicate to invasive species, de- cant component of global changes, connected with human spite that fact scientist still try to find the answer for the activity (Vitousek et al. 1997; Vilá et al. 2007; McKinney question: what characteristics make them invasive? (Rej- 2006). mánek 1995). The major point of the biological invasions was the The article is dedicated to the Fallopia genus, whose discovery of America by Christopher Columbus in 1492, some species are known as successful invaders, wide which has facilitated exchange of goods between new and spread in Europe and North America. -

Fallopia Japonica – Japanese Knotweed
Fallopia japonica – Japanese knotweed Japanese knotweed, sometimes referred to What is it? as donkey rhubarb for its sour red spring shoots, is a perennial plant in the Buckwheat family (Polygonaceae). It has large broad green leaves; tall, thick, sectioned and somewhat reddish zigzagging stems; and racemes of small papery flowers in summer. Photo by Liz West 2007 Other scientific names (synonyms) for Japanese knotweed are Reynoutria japonica and Polygonum cuspidatum. When does it grow? Shoots emerge from rhizomes (modified underground stems) from late March to mid-April. A spring freeze or deep frost can top kill new growth, but new shoots readily crop up from the hardy rootstalks. Growth continues rapidly once the weather begins to warm reaching heights up to 10 feet or greater by summer. R. Buczynski 2020 4.15.2020 Where is it from? Japanese knotweed is native to eastern Asia and was introduced to the United Kingdom in the 1800’s as a vigorous garden ornamental. Before becoming illegal to plant in England it was horticulturally introduced from the UK to the United States. Where is it now? Japanese knotweed has been reported extensively in the Northeastern U. S. and is currently present in all three counties (Hunterdon, Morris, and Somerset) within the upper Raritan watershed where it continues to spread into moist disturbed areas along waterways. Photo by Roger Kidd © Why is it invasive? Although knotweed can spread by seed, it is most effective at spreading underground via rhizomes that extend outward as well as downward, producing new shoots up to 70 feet away. If detached from the plant, small fragments of rhizome can survive and produce new plants wherever they land. -

Pocket Guide for Western North Carolina Partnership (SACWMP), 2011
DO NOT BUY Invasive Exotic Plant List Produced by the Southern Appalachian Cooperative Weed Management pocket guide for western north carolina Partnership (SACWMP), 2011 Western North Carolina has to offer! offer! to has Carolina North Western ) allegheniensis Rubus do not buy these invasives buy natives or alternatives ( Blackberry Allegheny ) alba Quercus ( Oak White of beautiful native plants that that plants native beautiful of ! Mimosa (Silk Tree) Albizia julibrissin Common Serviceberry (Amelanchier arborea) ) nigra Juglans ( Walnut Black Eastern Eastern Redbud (Cercis canadensis) multitude the enjoy and environment, To use your pocket guide: ) virginiana Diospyros ( Persimmon Flowering Dogwood (Cornus florida) whole the of quality the to Add counts. ) pumila Castanea ( Chinquapin the environment a favor on both both on favor a environment the 1 Print on letter-size paper. Japanese Barberry Berberis thunbergii Mountain Pepperbush (Clethra acuminata) wildlife for great Virginia Sweetspire (Itea virginica) doing are you plants, native planting Spicebush (Lindera benzoin) By habitat. species’ of loss the and 2 Cut along outer black line. are the spread of invasive exotic plants plants exotic invasive of spread the are ) fistulosum Eupatorium Butterfly Bush Buddleia davidii Swamp Milkweed (Asclepias incarnata) ( Weed Pye Joe ) ) purpurea (Echinacea Coneflower Purple Purple Coneflower (Echinacea purpurea) Carolina North Western in problems 3 Fold on dotted blue lines. ) syriaca Asclepias ( Milkweed Common Joe Pye Weed (Eupatorium fistulosum) -

Non-Native Invasive Plants of the City of Alexandria, Virginia
March 1, 2019 Non-Native Invasive Plants of the City of Alexandria, Virginia Non-native invasive plants have increasingly become a major threat to natural areas, parks, forests, and wetlands by displacing native species and wildlife and significantly degrading habitats. Today, they are considered the greatest threat to natural areas and global biodiversity, second only to habitat loss resulting from development and urbanization (Vitousek et al. 1996, Pimentel et al. 2005). The Virginia Department of Conservation and Recreation has identified 90 non-native invasive plants that threaten natural areas and lands in Virginia (Heffernan et al. 2014) and Swearingen et al. (2010) include 80 plants from a list of nearly 280 non-native invasive plant species documented within the mid- Atlantic region. Largely overlapping with these and other regional lists are 116 species that were documented in the City of Alexandria, Virginia during vegetation surveys and natural resource assessments by the City of Alexandria Dept. of Recreation, Parks, and Cultural Activities (RPCA), Natural Lands Management Section. This list is not regulatory but serves as an educational reference informing those with concerns about non-native invasive plants in the City of Alexandria and vicinity, including taking action to prevent the further spread of these species by not planting them. Exotic species are those that are not native to a particular place or habitat as a result of human intervention. A non-native invasive plant is here defined as one that exhibits some degree of invasiveness, whether dominant and widespread in a particular habitat or landscape or much less common but long-lived and extremely persistent in places where it occurs.