Directional Selection and Variation in Finite Populations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Response to Selection in Finite Locus Models with Nonadditive Effects
Journal of Heredity, 2017, 318–327 doi:10.1093/jhered/esw123 Original Article Advance Access publication January 12, 2017 Original Article Response to Selection in Finite Locus Models with Nonadditive Effects Hadi Esfandyari, Mark Henryon, Peer Berg, Jørn Rind Thomasen, Downloaded from https://academic.oup.com/jhered/article/108/3/318/2895120 by guest on 23 September 2021 Piter Bijma, and Anders Christian Sørensen From the Center for Quantitative Genetics and Genomics, Department of Molecular Biology and Genetics, Aarhus University DK-8830 Tjele, Denmark (Esfandyari, Berg, Thomasen, and Sørensen); Seges, Danish Pig Research Centre, Copenhagen, Denmark (Henryon); School of Animal Biology, University of Western Australia, Crawley, Australia (Henryon); Nordic Genetic Resource Center, Ås, Norway (Berg); VikingGenetics, Assentoft, Denmark (Thomasen); and Animal Breeding and Genomics Centre, Wageningen University, Wageningen, The Netherlands (Bijma). Address correspondence to H. Esfandyari at the address above, or e-mail: hadi.esfandyari@ mbg.au.dk. Received May 18, 2016; First decision June 7, 2016; Accepted January 2, 2017. Corresponding Editor: C Scott Baker Abstract Under the finite-locus model in the absence of mutation, the additive genetic variation is expected to decrease when directional selection is acting on a population, according to quantitative-genetic theory. However, some theoretical studies of selection suggest that the level of additive variance can be sustained or even increased when nonadditive genetic effects are present. We tested the hypothesis that finite-locus models with both additive and nonadditive genetic effects maintain more additive genetic variance (VA) and realize larger medium- to long-term genetic gains than models with only additive effects when the trait under selection is subject to truncation selection. -

Selection Response in Finite Populations
Copyright 0 1996 by the Genetics Society of America Selection Responsein Finite Populations Ming Wei, Armando Caballero and William G. Hill Institute of Cell, Animal and Population Biology, University of Edinburgh, King’s Buildings, Edinburgh EH9 3JT, United Kingdom Manuscript received November 28, 1995 Accepted for publication September 5, 1996 ABSTRACT Formulae were derived to predict genetic response under various selection schemes assuming an infinitesimal model. Accountwas taken of genetic drift, gametic (linkage) disequilibrium (Bulmereffect), inbreeding depression, common environmental variance, and both initial segregating variance within families (a;wo)and mutational (&) variance. The cumulative response to selection until generation t(C8) can be approximated as where N, is the effective population size, = Ne& is the genetic variancewithin families at the steady state (or one-half the genic variance, which is unaffected by selection), and D is the inbreeding depression per unit of inbreeding. & is the selection response at generation 0 assuming preselection so that the linkage disequilibrium effect has stabilized. P is the derivative of the logarithm of the asymptotic response with respect to the logarithm of the within-family genetic variance, ie., their relative rate of change. & is the major determinant of the short term selection response, but aL, Ne and p are also important for the long term. A selection method of high accuracy using family information gives a small N, and will lead to a larger- response in the short term and a smaller response in the long term, utilizing mutation less efficiently. ENETIC response to one cycle of selection for a 1975) using all information available are formally the G quantitative character is solely a function of selec- methods to achieve the maximum response for one tion accuracy (the correlation between the selection generation of selection. -

The Qtn Program and the Alleles That Matter for Evolution: All That’S Gold Does Not Glitter
PERSPECTIVE doi:10.1111/j.1558-5646.2011.01486.x THE QTN PROGRAM AND THE ALLELES THAT MATTER FOR EVOLUTION: ALL THAT’S GOLD DOES NOT GLITTER Matthew V. Rockman1,2 1Department of Biology and Center for Genomics and Systems Biology, New York University, 12 Waverly Place, New York, NY 10003 2E-mail: [email protected] Received April 20, 2011 Accepted September 30, 2011 The search for the alleles that matter, the quantitative trait nucleotides (QTNs) that underlie heritable variation within populations and divergence among them, is a popular pursuit. But what is the question to which QTNs are the answer? Although their pursuit is often invoked as a means of addressing the molecular basis of phenotypic evolution or of estimating the roles of evolutionary forces, the QTNs that are accessible to experimentalists, QTNs of relatively large effect, may be uninformative about these issues if large-effect variants are unrepresentative of the alleles that matter. Although 20th century evolutionary biology generally viewed large-effect variants as atypical, the field has recently undergone a quiet realignment toward a view of readily discoverable large- effect alleles as the primary molecular substrates for evolution. I argue that neither theory nor data justify this realignment. Models and experimental findings covering broad swaths of evolutionary phenomena suggest that evolution often acts via large numbers of small-effect polygenes, individually undetectable. Moreover, these small-effect variants are different in kind, at the molecular level, from the large-effect alleles accessible to experimentalists. Although discoverable QTNs address some fundamental evolutionary questions, they are essentially misleading about many others. -

Patterns and Power of Phenotypic Selection in Nature
Articles Patterns and Power of Phenotypic Selection in Nature JOEL G. KINGSOLVER AND DAVID W. PFENNIG Phenotypic selection occurs when individuals with certain characteristics produce more surviving offspring than individuals with other characteristics. Although selection is regarded as the chief engine of evolutionary change, scientists have only recently begun to measure its action in the wild. These studies raise numerous questions: How strong is selection, and do different types of traits experience different patterns of selection? Is selection on traits that affect mating success as strong as selection on traits that affect survival? Does selection tend to favor larger body size, and, if so, what are its consequences? We explore these questions and discuss the pitfalls and future prospects of measuring selection in natural populations. Keywords: adaptive landscape, Cope’s rule, natural selection, rapid evolution, sexual selection henotypic selection occurs when individuals with selection on traits that affect survival stronger than on those Pdifferent characteristics (i.e., different phenotypes) that affect only mating success? In this article, we explore these differ in their survival, fecundity, or mating success. The idea and other questions about the patterns and power of phe- of phenotypic selection traces back to Darwin and Wallace notypic selection in nature. (1858), and selection is widely accepted as the primary cause of adaptive evolution within natural populations.Yet Darwin What is selection, and how does it work? never attempted to measure selection in nature, and in the Selection is the nonrandom differential survival or repro- century following the publication of On the Origin of Species duction of phenotypically different individuals. -

Selection and Gene Duplication: a View from the Genome Comment Andreas Wagner
http://genomebiology.com/2002/3/5/reviews/1012.1 Minireview Selection and gene duplication: a view from the genome comment Andreas Wagner Address: Department of Biology, University of New Mexico, 167A Castetter Hall, Albuquerque, NM 817131-1091, USA. E-mail: [email protected] Published: 15 April 2002 Genome Biology 2002, 3(5):reviews1012.1–1012.3 reviews The electronic version of this article is the complete one and can be found online at http://genomebiology.com/2002/3/5/reviews/1012 © BioMed Central Ltd (Print ISSN 1465-6906; Online ISSN 1465-6914) Abstract reports Immediately after a gene duplication event, the duplicate genes have redundant functions. Is natural selection therefore completely relaxed after duplication? Does one gene evolve more rapidly than the other? Several recent genome-wide studies have suggested that duplicate genes are always under purifying selection and do not always evolve at the same rate. deposited research When a gene duplication event occurs, the duplicate genes synonymous (silent) nucleotide substitutions, Ks, and have redundant functions. Many deleterious mutations may second, of non-synonymous nucleotide substitutions (which then be harmless, because even if one gene suffers a muta- change the encoded amino acid), Ka (see Box 1). The ratio tion, the redundant gene copy can provide a back-up func- Ka/Ks provides a measure of the selection pressure to which tion. Put differently, after gene duplication - which can arise a gene pair is subject. If a duplicate gene pair shows a Ka/Ks through polyploidization (whole-genome duplication), non- ratio of about 1, that is, if amino-acid replacement substitu- homologous recombination, or through the action of retro- tions occur at the same rate as synonymous substitutions, transposons - one or both duplicates should experience then few or no amino-acid replacement substitutions have refereed research relaxed selective constraints that result in elevated rates of been eliminated since the gene duplication. -

Alleles Versus Mutations: Understanding the Evolution of Genetic Architecture Requires a Molecular Perspective on Allelic Origins
Alleles versus mutations: Understanding the evolution of genetic architecture requires a molecular perspective on allelic origins By: David L. Remington Remington, D. L. (2015). Alleles versus mutations: understanding the evolution of genetic architecture requires a molecular perspective on allelic origins. Evolution: international journal of organic evolution. doi:10.1111/evo.12775 "This is the peer reviewed version of the following article: Remington, D. L. (2015). Alleles versus mutations: understanding the evolution of genetic architecture requires a molecular perspective on allelic origins. Evolution: international journal of organic evolution. doi:10.1111/evo.12775, which has been published in final form at http://dx.doi.org/10.1111/evo.12775. This article may be used for non-commercial purposes in accordance with Wiley Terms and Conditions for Self-Archiving." Abstract: Perspectives on the role of large-effect quantitative trait loci (QTL) in the evolution of complex traits have shifted back and forth over the past few decades. Different sets of studies have produced contradictory insights on the evolution of genetic architecture. I argue that much of the confusion results from a failure to distinguish mutational and allelic effects, a limitation of using the Fisherian model of adaptive evolution as the lens through which the evolution of adaptive variation is examined. A molecular-based perspective reveals that allelic differences can involve the cumulative effects of many mutations plus intragenic recombination, a model that is supported by extensive empirical evidence. I discuss how different selection regimes could produce very different architectures of allelic effects under a molecular-based model, which may explain conflicting insights on genetic architecture from studies of variation within populations versus between divergently selected populations. -
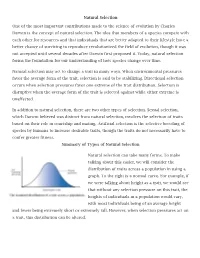
Natural Selection One of the Most Important Contributions Made to The
Natural Selection One of the most important contributions made to the science of evolution by Charles Darwin is the concept of natural selection. The idea that members of a species compete with each other for resources and that individuals that are better adapted to their lifestyle have a better chance of surviving to reproduce revolutionized the field of evolution, though it was not accepted until several decades after Darwin first proposed it. Today, natural selection forms the foundation for our understanding of how species change over time. Natural selection may act to change a trait in many ways. When environmental pressures favor the average form of the trait, selection is said to be stabilizing. Directional selection occurs when selection pressures favor one extreme of the trait distribution. Selection is disruptive when the average form of the trait is selected against while either extreme is unaffected. In addition to natural selection, there are two other types of selection. Sexual selection, which Darwin believed was distinct from natural selection, involves the selection of traits based on their role in courtship and mating. Artificial selection is the selective breeding of species by humans to increase desirable traits, though the traits do not necessarily have to confer greater fitness. Summary of Types of Natural Selection Natural selection can take many forms. To make talking about this easier, we will consider the distribution of traits across a population in using a graph. To the right is a normal curve. For example, if we were talking about height as a trait, we would see that without any selection pressure on this trait, the heights of individuals in a population would vary, with most individuals being of an average height and fewer being extremely short or extremely tall. -

Detecting the Form of Selection from DNA Sequence Data
Outlook COMMENT Male:female mutation ratio muscular dystrophy, neurofibromatosis and retinoblast- outcome of further investigation, this unique transposition oma), there is a large male excess. So, I believe the evidence event and perhaps others like it are sure to provide impor- is convincing that the male base-substitution rate greatly tant cytogenetic and evolutionary insights. exceeds the female rate. There are a number of reasons why the historical Acknowledgements male:female mutation ratio is likely to be less than the I have profited greatly from discussions with my colleagues current one, the most important being the lower age of B. Dove, B. Engels, C. Denniston and M. Susman. My reproduction in the earliest humans and their ape-like greatest debt is to D. Page for a continuing and very fruitful ancestors. More studies are needed. But whatever the dialog and for providing me with unpublished data. References Nature 406, 622–625 6 Engels, W.R. et al. (1994) Long-range cis preference in DNA 1 Vogel, F. and Motulsky, A. G. (1997) Human Genetics: 4 Shimmin, L.C. et al. (1993) Potential problems in estimating homology search over the length of a Drosophila chromosome. Problems and Approaches. Springer-Verlag the male-to-female mutation rate ratio from DNA sequence Science 263, 1623–1625 2 Crow, J. F. (1997) The high spontaneous mutation rate: is it a data. J. Mol. Evol. 37, 160–166 7 Crow, J.F. (2000) The origins, patterns and implications of health risk? Proc. Natl. Acad. Sci. U. S. A. 94, 8380–8386 5 Richardson, C. et al. -

100 Years of Quantitative Genetics Theory and Its Applications: Celebrating the Centenary of Fisher 1918
100 years of quantitative genetics theory and its applications: celebrating the centenary of Fisher 1918 Nick Barton Institute for Science and Technology, Klosterneuburg, Austria The infinitesimal model In his 1918 paper, Fisher introduced the analysis of variance, and reconciled Mendelian genetics with continuous biometric variation. He also showed that when variation is due to very many additive genetic loci, selection hardly changes the genetic variance. This has come to be known as the “infinitesimal model”, and is the foundation for practical animal breeding. Yet, organisms are plainly not simply the sum of their genes, and so it is not clear when the use of the infinitesimal model can be justified. I review the history of the infinitesimal model, and show how it can be extended to include dominance, epistasis, and linkage. Sharon Browning University of Washington, Seattle, Washington, USA Identity by descent and the correlation between distant relatives Identity by descent and the correlation between distant relatives Related individuals have correlated trait values because they share parts of their genomes identical by descent. Because the genome is inherited in large chunks, punctuated by crossovers, the identity by descent (IBD) occurs in long segments. IBD segments inherited from more distant ancestors tend to be shorter due to more opportunities for crossing over. So-called “unrelated” individuals are in fact distant relatives, and share short segments of IBD. I will describe the detection of segments of IBD in population samples using genome-wide genetic data, and the application of these segments to IBD mapping and to estimating heritability. Edward Buckler USDA-ARS, Ithaca, New York, USA How to get to Plant Breeding 4.0, given that Fisher was right? As global population continues to grow and standards of living rise, the world food supply needs to almost double protein production. -

Animal Breeding from Infinitesimal Model to Mas: the Case of a Backcross Design in Dairy Sheep (Sarda X Lacaune) and Its Possible Impact on Selection
MARKER ASSISTED SELECTION: A FAST TRACK TO INCREASE GENETIC GAIN IN PLANT AND ANIMAL BREEDING? SESSION II: MAS IN ANIMALS ANIMAL BREEDING FROM INFINITESIMAL MODEL TO MAS: THE CASE OF A BACKCROSS DESIGN IN DAIRY SHEEP (SARDA X LACAUNE) AND ITS POSSIBLE IMPACT ON SELECTION G. Pagnacco and A. Carta [email protected] [email protected] Summary Despite the great advancements achieved by quantitative techniques, selection in some species and for some traits, as in dairy sheep, can be very expensive and requires great efforts. In this paper we review the options offered by quantitative methods and by marker-assisted selection and we discuss the possible impact of a QTL detection design based on a backcross experimental population (Sarda X Lacaune) on sheep breeding. Keywords Infinitesimal model, Quantitative Trait Loci, Marker-assisted selection, dairy sheep, Backcross Design Abstract Selection under the infinitesimal model Standard animal breeding techniques have been largely successful in improving the performance of domestic animals in the last century. The success of these techniques finds its roots in the theoretical work of Fisher and Wright who showed how selection, migration and mutation could be derived from the simple Mendelian laws. All that theory was then transferred into the operational application in animal breeding by J. L. Lush (Animal Breeding Plans, 1945) and by his many “sons” and “grandsons”. In more recent times another landmark book, Introduction to Quantitative Genetics, (Falconer, 1960) probably represents the official birth of the quantitative genetics in plant and especially animal breeding. Plant and animal breeding have based their tools of investigation on this “central paradigm for the analysis of phenotypic variation” (Lynch and Walsh, 1998) even if their methods have radically diverged probably because of the different population structure in crop and domestic animals. -

The Peppered Moth and Industrial Melanism: Evolution of a Natural Selection Case Study
Heredity (2013) 110, 207–212 & 2013 Macmillan Publishers Limited All rights reserved 0018-067X/13 www.nature.com/hdy REVIEW The peppered moth and industrial melanism: evolution of a natural selection case study LM Cook1 and IJ Saccheri2 From the outset multiple causes have been suggested for changes in melanic gene frequency in the peppered moth Biston betularia and other industrial melanic moths. These have included higher intrinsic fitness of melanic forms and selective predation for camouflage. The possible existence and origin of heterozygote advantage has been debated. From the 1950s, as a result of experimental evidence, selective predation became the favoured explanation and is undoubtedly the major factor driving the frequency change. However, modelling and monitoring of declining melanic frequencies since the 1970s indicate either that migration rates are much higher than existing direct estimates suggested or else, or in addition, non-visual selection has a role. Recent molecular work on genetics has revealed that the melanic (carbonaria) allele had a single origin in Britain, and that the locus is orthologous to a major wing patterning locus in Heliconius butterflies. New methods of analysis should supply further information on the melanic system and on migration that will complete our understanding of this important example of rapid evolution. Heredity (2013) 110, 207–212; doi:10.1038/hdy.2012.92; published online 5 December 2012 Keywords: Biston betularia; carbonaria gene; mutation; predation; non-visual selection; migration INTRODUCTION EARLY EVIDENCE OF CHANGE The peppered moth Biston betularia (L.) and its melanic mutant will The peppered moth was the most diagrammatic example of the be familiar to readers of Heredity as an example of rapid evolutionary phenomenon of industrial melanism that came to be recognised in change brought about by natural selection in a changing environment, industrial and smoke-blackened parts of England in the mid-nine- evenifthedetailsofthestoryarenot.Infact,thedetailsarelesssimple teenth century. -

Natural Selection in a Contemporary Human Population
Natural selection in a contemporary human population Sean G. Byarsa, Douglas Ewbankb, Diddahally R. Govindarajuc, and Stephen C. Stearnsa,1 aDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520-8102; bPopulation Studies Center, University of Pennsylvania, Philadelphia, PA 19104-6299; and cDepartment of Neurology, Boston University School of Medicine, Boston, MA 02118-2526 Edited by Peter T. Ellison, Harvard University, Cambridge, MA, and approved September 16, 2009 (received for review June 25, 2009) Our aims were to demonstrate that natural selection is operating sity to identify factors that contribute to cardiovascular disease. on contemporary humans, predict future evolutionary change for It is the longest running multigenerational study in medical specific traits with medical significance, and show that for some history. The people originally enrolled in the study were of pre- traits we can make short-term predictions about our future evolu- dominantly European ancestry (20% United Kingdom, 40% tion. To do so, we measured the strength of selection, estimated Ireland, 10% Italy, 10% Quebec). The original cohort (n = genetic variation and covariation, and predicted the response to 5,209) has been examined every 2 years, a total of 29 times selection for women in the Framingham Heart Study, a project of between 1948 and 2008. The offspring cohort (n = 5,124) has the National Heart, Lung, and Blood Institute and Boston Univer- been examined approximately every 4 years, a total of eight times sity that began in 1948. We found that natural selection is acting between 1971 and 2008 (4). There is also a third generation to cause slow, gradual evolutionary change.