Low-Dose Imipramine for Refractory Functional Dyspepsia: a Randomised, Double-Blind, Placebo-Controlled Trial
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Developments in Prokinetic Therapy for Gastric Motility Disorders
REVIEW published: 24 August 2021 doi: 10.3389/fphar.2021.711500 New Developments in Prokinetic Therapy for Gastric Motility Disorders Michael Camilleri* and Jessica Atieh Clinical Enteric Neuroscience Translational and Epidemiological Research (CENTER), Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States Prokinetic agents amplify and coordinate the gastrointestinal muscular contractions to facilitate the transit of intra-luminal content. Following the institution of dietary recommendations, prokinetics are the first medications whose goal is to improve gastric emptying and relieve symptoms of gastroparesis. The recommended use of metoclopramide, the only currently approved medication for gastroparesis in the United States, is for a duration of less than 3 months, due to the risk of reversible or irreversible extrapyramidal tremors. Domperidone, a dopamine D2 receptor antagonist, is available for prescription through the FDA’s program for Expanded Access to Investigational Drugs. Macrolides are used off label and are associated with tachyphylaxis and variable duration of efficacy. Aprepitant relieves some symptoms of gastroparesis. There are newer agents in the pipeline targeting diverse gastric (fundic, antral and pyloric) motor functions, including novel serotonergic 5-HT4 agonists, dopaminergic D2/3 antagonists, neurokinin NK1 antagonists, and ghrelin agonist. Novel Edited by: targets with potential to improve gastric motor functions include the pylorus, macrophage/ Jan Tack, inflammatory function, oxidative -

Prokinetics and Ghrelin for the Management of Cancer Cachexia Syndrome
85 Review Article Prokinetics and ghrelin for the management of cancer cachexia syndrome Jimi S. Malik, Sriram Yennurajalingam Department of Palliative Care, Rehabilitation and Integrative Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA Contributions: (I) Conception and design: All authors; (II) Administrative support: S Yennurajalingam; (III) Provision of study materials or patients: S Yennurajalingam; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Sriram Yennurajalingam, MD. Department of Palliative Care, Rehabilitation, and Integrative Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1414, Houston, TX 77030, USA. Email: [email protected]. Abstract: Cancer cachexia (CC) is one of the most distressing syndromes for both patients and their families. CC can have an impact on patient reported quality of life and overall survival. It is often associated with symptoms such as fatigue, depressed mood, early satiety, and anorexia. Prokinetic agents have been found to improve chronic nausea and early satiety associated with CC. Among the prokinetic agents, metoclopramide is one of the best studied medications. The role of the other prokinetic agents, such as domperidone, erythromycin, haloperidol, levosulpiride, tegaserod, cisapride, mosapride, renzapride, and prucalopride is unclear for use in cachectic cancer patients due to their side effect profile and limited efficacy studies in cancer patients. There has been an increased interest in the use of ghrelin-receptor agonists for the treatment of CC. Anamorelin HCl is a highly selective, novel ghrelin receptor agonist. -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

Drugs That Can Cause Delirium (Anticholinergic / Toxic Metabolites)
Drugs that can Cause Delirium (anticholinergic / toxic metabolites) Deliriants (drugs causing delirium) Prescription drugs . Central acting agents – Sedative hypnotics (e.g., benzodiazepines) – Anticonvulsants (e.g., barbiturates) – Antiparkinsonian agents (e.g., benztropine, trihexyphenidyl) . Analgesics – Narcotics (NB. meperidine*) – Non-steroidal anti-inflammatory drugs* . Antihistamines (first generation, e.g., hydroxyzine) . Gastrointestinal agents – Antispasmodics – H2-blockers* . Antinauseants – Scopolamine – Dimenhydrinate . Antibiotics – Fluoroquinolones* . Psychotropic medications – Tricyclic antidepressants – Lithium* . Cardiac medications – Antiarrhythmics – Digitalis* – Antihypertensives (b-blockers, methyldopa) . Miscellaneous – Skeletal muscle relaxants – Steroids Over the counter medications and complementary/alternative medications . Antihistamines (NB. first generation) – diphenhydramine, chlorpheniramine). Antinauseants – dimenhydrinate, scopolamine . Liquid medications containing alcohol . Mandrake . Henbane . Jimson weed . Atropa belladonna extract * Requires adjustment in renal impairment. From: K Alagiakrishnan, C A Wiens. (2004). An approach to drug induced delirium in the elderly. Postgrad Med J, 80, 388–393. Delirium in the Older Person: A Medical Emergency. Island Health www.viha.ca/mhas/resources/delirium/ Drugs that can cause delirium. Reviewed: 8-2014 Some commonly used medications with moderate to high anticholinergic properties and alternative suggestions Type of medication Alternatives with less deliriogenic -

Cabergoline Patient Handout
Cabergoline For the Patient: Cabergoline Other names: DOSTINEX® • Cabergoline (ca-BERG-go-leen) is used to treat cancers that cause the body to produce too much of a hormone called prolactin. Cabergoline helps decrease the size of the cancer and the production of prolactin. It is a tablet that you take by mouth. • Tell your doctor if you have ever had an unusual or allergic reaction to bromocriptine or other ergot derivatives, such as pergoline (PERMAX®) and methysergide (SANSERT®), before taking cabergoline. • Blood tests and blood pressure measurement may be taken while you are taking cabergoline. The dose of cabergoline may be changed based on the test results and/or other side effects. • It is important to take cabergoline exactly as directed by your doctor. Make sure you understand the directions. Take cabergoline with food. • If you miss a dose of cabergoline, take it as soon as you can if it is within 2 days of the missed dose. If it is over 2 days since your missed dose, skip the missed dose and go back to your usual dosing times. • Other drugs such as azithromycin (ZITHROMAX®), clarithromycin (BIAXIN®), erythromycin, domperidone, metoclopramide, and some drugs used to treat mental or mood problems may interact with cabergoline. Tell your doctor if you are taking these or any other drugs as you may need extra blood tests or your dose may need to be changed. Check with your doctor or pharmacist before you start or stop taking any other drugs. • The drinking of alcohol (in small amounts) does not appear to affect the safety or usefulness of cabergoline. -
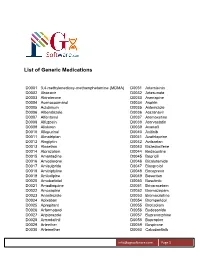
List of Generic Medications
List of Generic Medications D0001 3,4-methylenedioxy-methamphetamine (MDMA) D0031 Artemisinin D0002 Abacavir D0032 Artesunate D0003 Abiraterone D0033 Asenapine D0004 Acenocoumarol D0034 Aspirin D0005 Aclidinium D0035 Astemizole D0006 Albendazole D0036 Atazanavir D0007 Alfentanyl D0037 Atomoxetine D0008 Alfuzosin D0038 Atorvastatin D0009 Aliskiren D0039 Avanafil D0010 Allopurinol D0040 Axitinib D0011 Almotriptan D0041 Azathioprine D0012 Alogliptin D0042 Azilsartan D0013 Alosetron D0043 Bazedoxifene D0014 Alprazolam D0044 Bedaquiline D0015 Amantadine D0045 Bepridil D0016 Amiodarone D0046 Bicalutamide D0017 Amisulpride D0047 Bisoprolol D0018 Amitriptyline D0048 Boceprevir D0019 Amlodipine D0049 Bosentan D0020 Amobarbital D0050 Bosutinib D0021 Amodiaquine D0051 Brivaracetam D0022 Amoxapine D0052 Bromazepam D0023 Anastrozole D0053 Bromocriptine D0024 Apixaban D0054 Bromperidol D0025 Aprepitant D0055 Brotizolam D0026 Arformoterol D0056 Budesonide D0027 Aripiprazole D0057 Buprenorphine D0028 Armodafinil D0058 Bupropion D0029 Arteether D0059 Buspirone D0030 Artemether D0060 Cabozantinib [email protected] Page 1 List of Generic Medications D0061 Canagliflozin D0091 Clofarabine D0062 Cannabidiol (CBD) D0092 Clofibrate D0063 Cannabinol (CBN) D0093 Clomipramine D0064 Captopril D0094 Clonazepam D0065 Carbamazepine D0095 Clonidine D0066 Carisoprodol D0096 Clopidogrel D0067 Carmustine D0097 Clorazepate D0068 Carvedilol D0098 Clozapine D0069 Celecoxib D0099 Cocaine D0070 Ceritinib D0100 Codeine D0071 Cerivastatin D0101 Colchicine D0072 Cetirizine -

Gastroparesis: 2014
GASTROINTESTINAL MOTILITY AND FUNCTIONAL BOWEL DISORDERS, SERIES #1 Richard W. McCallum, MD, FACP, FRACP (Aust), FACG Status of Pharmacologic Management of Gastroparesis: 2014 Richard W. McCallum Joseph Sunny, Jr. Gastroparesis is characterized by delayed gastric emptying without mechanical obstruction of the gastric outlet or small intestine. The main etiologies are diabetes, idiopathic and post- gastric and esophageal surgical settings. The management of gastroparesis is challenging due to a limited number of medications and patients often have symptoms, which are refractory to available medications. This article reviews current treatment options for gastroparesis including adverse events and limitations as well as future directions in pharmacologic research. INTRODUCTION astroparesis is a syndrome characterized by documented gastroparesis are increasing.2 Physicians delayed emptying of gastric contents without have both medical and surgical approaches for these Gmechanical obstruction of the stomach, pylorus or patients (See Figure 1). Medical therapy includes both small bowel. Patients can present with nausea, vomiting, prokinetics and antiemetics (See Table 1 and Table 2). postprandial fullness, early satiety, pressure, fullness The gastroparesis population will grow as diabetes and abdominal distension. In addition, abdominal pain increases and new therapies will be required. What located in the epigastrium, and distinguished from the do we know about the size of the gastroparetic term discomfort, is increasingly being recognized population? According to a study from the Mayo Clinic as an important symptom. The main etiologies of group surveying Olmsted County in Minnesota, the gastroparesis are diabetes, idiopathic, and post gastric risk of gastroparesis in Type 1 diabetes mellitus was and esophageal surgeries.1 Hospitalizations from significantly greater than for Type 2. -

A Four-Country Comparison of Healthcare Systems, Implementation
Neurogastroenterology & Motility Neurogastroenterol Motil (2014) 26, 1368–1385 doi: 10.1111/nmo.12402 REVIEW ARTICLE A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders A report of the Rome Foundation Working Team on cross-cultural, multinational research M. SCHMULSON,* E. CORAZZIARI,† U. C. GHOSHAL,‡ S.-J. MYUNG,§ C. D. GERSON,¶ E. M. M. QUIGLEY,** K.-A. GWEE†† & A. D. SPERBER‡‡ *Laboratorio de Hıgado, Pancreas y Motilidad (HIPAM)-Department of Experimental Medicine, Faculty of Medicine-Universidad Nacional Autonoma de Mexico (UNAM). Hospital General de Mexico, Mexico City, Mexico †Gastroenterologia A, Department of Internal Medicine and Medical Specialties, University La Sapienza, Rome, Italy ‡Department of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Science, Lucknow, India §Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea ¶Division of Gastroenterology, Mount Sinai School of Medicine, New York, NY, USA **Division of Gastroenterology and Hepatology, Houston Methodist Hospital and Weill Cornell Medical College, Houston, TX, USA ††Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore ‡‡Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel Key Messages • This report identified seven key issues related to healthcare provision that may impact how patients with FGIDs are investigated, diagnosed and managed. • Variations in healthcare provision around the world in patients with FGIDs have not been reviewed. • We compared four countries that are geographically and culturally diverse, and exhibit differences in the healthcare coverage provided to their population: Italy, South Korea, India and Mexico. • Since there is a paucity of publications relating to the issues covered in this report, some of the findings are based on the authors’ personal perspectives, press reports and other published sources. -

PRESCRIBED DRUGS and NEUROLOGICAL COMPLICATIONS K a Grosset, D G Grosset Iii2
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.045757 on 16 August 2004. Downloaded from PRESCRIBED DRUGS AND NEUROLOGICAL COMPLICATIONS K A Grosset, D G Grosset iii2 J Neurol Neurosurg Psychiatry 2004;75(Suppl III):iii2–iii8. doi: 10.1136/jnnp.2004.045757 treatment history is a fundamental part of the healthcare consultation. Current drugs (prescribed, over the counter, herbal remedies, drugs of misuse) and how they are taken A(frequency, timing, missed and extra doses), drugs tried previously and reason for discontinuation, treatment response, adverse effects, allergies, and intolerances should be taken into account. Recent immunisations may also be of importance. This article examines the particular relevance of medication in patients presenting with neurological symptoms. Drugs and their interactions may contribute in part or fully to the neurological syndrome, and treatment response may assist diagnostically or in future management plans. Knowledge of medicine taking behaviour may clarify clinical presentations such as analgesic overuse causing chronic daily headache, or severe dyskinesia resulting from obsessive use of dopamine replacement treatment. In most cases, iatrogenic symptoms are best managed by withdrawal of the offending drug. Indirect mechanisms whereby drugs could cause neurological problems are beyond the scope of the current article—for example, drugs which raise blood pressure or which worsen glycaemic control and consequently increase the risk of cerebrovascular disease, or immunosupressants -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

NEUROPHARMACOLOGICAL COMPARISON BETWEEN DOMPERIDONE and METOCLOPRAMIDE Abstract-Domperidone, a New Gastrokinetic with Potent
In in vitro binding assays, domperidone In in vitro experiments on the isolated was found to be a potent and specific guinea-pig stomach, domperidone selectively dopamine antagonist, but ex vivo and in vivo NEUROPHARMACOLOGICAL COMPARISON BETWEEN DOMPERIDONE AND METOCLOPRAMIDE A. WAUQUIER, C.J.E. NIEMEGEERS and P.A.J. JANSSEN Department of Pharmacology, Janssen Pharmaceutica , B-2340 Beerse, Belgium Accepted October 17, 1980 Abstract-Domperidone, a new gastrokinetic with potent antiemetic properties is devoid of central effects, up to high dose levels. To assess the CNS activity of domperidone and metoclopramide, the inhibition of intracranial self-stimulation (ICS) in two different situations, and the influence on EEG in dogs were studied. The dissociation between the antiemetic and central effects of both compounds were evaluated in dogs given a stereotypogenic dose of apomorphine. A significant and dose related inhibition of ICS (conditioned situation) was obtained with 0.8 and 1.6 mg/kg i.v. domperidone and with 0.125, 0.25 and 0.50 mg/kg i.v. metoclopramide. The ED50 values were 0.79 mg/kg and 0.25 mg/kg respectively. The effect was most pronounced 4 hr after administration with domperidone and 15 min after administration with metoclopramide. In the EEG studies, no specific structure-related effects were found but the total potency was increased with domperidone 0.8 and 1.6 mg/kg i.v. and with metoclopramide 0.063, 0.125, 0.25 and 0.50 mg/kg i.v. This increase was due to a decrease in fast frequencies, an increase of slower frequencies and a slight increase of the amplitude. -

A Systematic Review of the Effectiveness of Antianxiety and Antidepressive Agents for Functional Dyspepsia
doi: 10.2169/internalmedicine.9099-17 Intern Med 56: 3127-3133, 2017 http://internmed.jp 【 ORIGINAL ARTICLE 】 A Systematic Review of the Effectiveness of Antianxiety and Antidepressive Agents for Functional Dyspepsia Mariko Hojo 1, Akihito Nagahara 2, Daisuke Asaoka 1, Yuji Shimada 2, Hitoshi Sasaki 3, Kohei Matsumoto 1, Tsutomu Takeda 1, Hiroya Ueyama 1, Kenshi Matsumoto 1 and Sumio Watanabe 1 Abstract: Objective Functional dyspepsia (FD) is defined as persistent or recurrent pain or discomfort centered in the upper abdomen without organic disease. Psychosocial factors have been proposed as an important element in the pathophysiology of FD. Therefore, psychotropic agents having antianxiety or antidepressive action are ex- pected to alleviate FD. We previously reported on the treatment of FD using such agents in a systematic re- view, wherein the effectiveness of the agents on FD was suggested, although there were several limitations. We searched for articles on this subject after our systematic review and re-reviewed them systematically. Methods Articles were searched for in MEDLINE from 2003 to 2014 using terms related to antianxiety or antidepressive agents. Clinical studies in which the effectiveness of such agents was clearly stated were se- lected from the retrieved articles. The newly selected and previously selected studies were combined, and sta- tistical analyses were carried out. Results Nine studies were selected. Five of the studies indicated a significant symptomatic improvement us- ing psychotropic drugs. A statistical analysis suggested a significant treatment effect of psychotropic agents having antianxiety or antidepressive action [pooled relative risk (PRR), 0.72; 95% confidence interval (95% CI), 0.52-0.99; p=0.0406] but did not show a significant benefit of treatment with agents having an antide- pressive action alone (PRR, 0.63; 95% CI, 0.38-1.03; p=0.0665).