ESM 2. List of Most Threatened Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Anura: Hemiphractidae: Gastrotheca)
Accepted Manuscript Short communication Brazilian marsupial frogs are diphyletic (Anura: Hemiphractidae: Gastrotheca) David C. Blackburn, William E. Duellman PII: S1055-7903(13)00179-6 DOI: http://dx.doi.org/10.1016/j.ympev.2013.04.021 Reference: YMPEV 4580 To appear in: Molecular Phylogenetics and Evolution Received Date: 7 January 2013 Revised Date: 2 April 2013 Accepted Date: 22 April 2013 Please cite this article as: Blackburn, D.C., Duellman, W.E., Brazilian marsupial frogs are diphyletic (Anura: Hemiphractidae: Gastrotheca), Molecular Phylogenetics and Evolution (2013), doi: http://dx.doi.org/10.1016/ j.ympev.2013.04.021 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. 1 Short Communication 2 3 Brazilian marsupial frogs are diphyletic (Anura: Hemiphractidae: Gastrotheca) 4 5 David C. Blackburna,*, William E. Duellmanb 6 a Department of Vertebrate Zoology & Anthropology, California Academy of Sciences, 55 7 Music Concourse Drive, San Francisco, CA 94118, USA 8 b Biodiversity Institute, University of Kansas, 1345 Jayhawk Boulevard, Lawrence, KS 9 66045, USA 10 * Corresponding author. E-mail address: [email protected] (D.C. Blackburn) 11 12 Abstract 13 Molecular phylogenetic analyses based on expanded taxonomic and geographic sampling 14 support the monophyly of the marsupial frog genera (family Hemiphractidae), resolve six 15 geographically circumscribed lineages within Gastrotheca, and, for the first time, reveal 16 that two divergent lineages of Gastrotheca inhabit the Atlantic Coastal Forests of Brazil. -

Zootaxa, a New Species of Bryophryne (Anura
Zootaxa 1784: 1–10 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) A new species of Bryophryne (Anura: Strabomantidae) from southern Peru EDGAR LEHR1 & ALESSANDRO CATENAZZI2, 3 1Staatliche Naturhistorische Sammlungen Dresden, Museum für Tierkunde, Königsbrücker Landstrasse 159, D-01109 Dresden, Germany. E-mail: [email protected] 2Division of Integrative Biology, University of South Florida, 4202 East Fowler Ave, Tampa, FL 33620, USA 3Present address: Department of Integrative Biology, University of California at Berkeley. 3060 Valley Life Sciences Bldg #3140, Ber- keley CA 94720, USA. E-mail: [email protected] Abstract A new species of Bryophryne from southern Peru (Cusco Region) is described. Specimens were found in the leaf litter of cloud forest at elevations of 2350–3215 m. The new species has a maximum snout-vent length of 21.9 mm in adult females, 18.9 mm in adult males and is the smallest species of the genus. It lacks a tympanum and dentigerous processes of vomers, has dorsolateral folds, and males without vocal slits and without nuptial pads. The new species is most similar to B. bustamantei but differs in being smaller, having discontinuous dorsolateral folds, the males lacking vocal slits, and an overall darker ventral coloration. Bryophryne contains three species all of which lack a tympanum. The deep valley of the Río Apurímac as a distributional barrier separating Phrynopus from Bryophryne is discussed. Key words: Andes, biogeography, Bryophryne cophites, Bryophryne bustamantei Resumen Se describe una nueva especie de Bryophryne del sur de Perú (Región Cusco). -

Polyploidy and Sex Chromosome Evolution in Amphibians
Chapter 18 Polyploidization and Sex Chromosome Evolution in Amphibians Ben J. Evans, R. Alexander Pyron and John J. Wiens Abstract Genome duplication, including polyploid speciation and spontaneous polyploidy in diploid species, occurs more frequently in amphibians than mammals. One possible explanation is that some amphibians, unlike almost all mammals, have young sex chromosomes that carry a similar suite of genes (apart from the genetic trigger for sex determination). These species potentially can experience genome duplication without disrupting dosage stoichiometry between interacting proteins encoded by genes on the sex chromosomes and autosomalPROOF chromosomes. To explore this possibility, we performed a permutation aimed at testing whether amphibian species that experienced polyploid speciation or spontaneous polyploidy have younger sex chromosomes than other amphibians. While the most conservative permutation was not significant, the frog genera Xenopus and Leiopelma provide anecdotal support for a negative correlation between the age of sex chromosomes and a species’ propensity to undergo genome duplication. This study also points to more frequent turnover of sex chromosomes than previously proposed, and suggests a lack of statistical support for male versus female heterogamy in the most recent common ancestors of frogs, salamanders, and amphibians in general. Future advances in genomics undoubtedly will further illuminate the relationship between amphibian sex chromosome degeneration and genome duplication. B. J. Evans (CORRECTED&) Department of Biology, McMaster University, Life Sciences Building Room 328, 1280 Main Street West, Hamilton, ON L8S 4K1, Canada e-mail: [email protected] R. Alexander Pyron Department of Biological Sciences, The George Washington University, 2023 G St. NW, Washington, DC 20052, USA J. -

Species Limits, and Evolutionary History of Glassfrogs
!" # $"%!&"'(!$ ! )*)') !+ ,-.',)'**'-*)*' /0/ // ')11,2 !"#"$$$%$$& ' & & (' ') ' * ') + ,-'.)"$$). / 0 &1& )2 ) #3")44 ) )56,7,443,5474,3) 8 9 '' & ' & ' & ' * ) ' & ** ,& % & & & ' & ' ): '& ' ' ' '2 ) : ' ' ' ; < ;=2 > < ' * & &' '& ;& <) '' *'' & & ' &'' 9 * ' )? ' & ' & @ ' & ) ' '&' * & ' ' ;* ' '< &'>&' ) (' ' & 7$$ && ' ' ' & ' * ' ' )= &' & &*'' ' ) > * *& *'' ' ) : ' & & & ) > & 65 : , * A ) ' & & *' ' ' & & ' '= & ) 2 '2 ' & - ! (' = ( . . ! "# $ " # "% " "#!&'()* " B. + ,-'"$$ :..=7#47,#"73 :.=56,7,443,5474,3 % %%% ,7$$"C;' %AA )@)A D E % %%% ,7$$"C< Mathematical representation is inevitably simplistic, and occasionally one has to be brutal in forcing it to suit a reality that can only be very complex. And yet, there is a beauty about trees because of the simplicity with which they allow you to describe a series of events […]. But one must ask whether one is justified simplifying reality to the extent necessary to represent it as a tree. Cavalli-Sforza, Genes, People, and Languages (2001) The universe is no narrow thing and the order within it is not constrained by any latitude in is conception to repeat what exists in one part in any other part. Even in this world more things exist -

Notes on the Natural History of Three Glass Frogs Species (Anura: Centrolenidae) from the Andean Central Cordillera of Colombia*
BOLETÍN CIENTÍFICO ISSN 0123 - 3068 bol.cient.mus.hist.nat. 17 (2), julio - diciembre, 2013. 127 - 140 CENTRO DE MUSEOS MUSEO DE HISTORIA NATURAL NOTES ON THE NATURAL HISTORY OF THREE GLASS FROGS SPECIES (ANURA: CENTROLENIDAE) FROM THE ANDEAN CENTRAL CORDILLERA OF COLOMBIA* Julián Andrés Rojas-Morales1*;and Sergio Escobar-Lasso2 Abstract Between October 2008 and December 2010, we carried out surveys in different creeks at north of the municipality of Manizales in the Colombian Central Cordillera, in order to make observations on the natural history of three glass frogs species (Centrolene quindianum, Nymphargus grandisonae and N. spilotus). Centrolene quindianum presents clutches with 33–35 eggs (N= 2) located on the Upper of leaves at heights between 40–200 cm above water; one male was observed close to three egg-masses (< 1 m of distance of them in the same perch), suggesting the possibility of parental care. The spider Patrera armata (Anyphaenidae) is reported as a predator of the embryos of this species. In N. grandisonae both calling and oviposition occurs in the upper surfaces of large leaves, especially Araceas and Heliconias. These breeding sites are guarded territories by males for periods of up to six months. Their egg-masses have an average of 74 eggs (N = 4), located at a height and a distance from the edge of creeks between 57–218 cm (X = 113.21 ± 45.73 cm, N = 14) and 0–35 cm (X = 15.8 ± 6.7 cm, N = 14), respectively; males stay close to egg masses as they continue calling to attract females. In addition, we report for first timefly larvae (Drosophylidae) as parasitoid agent foregg-masses of N.grandisonae. -

Conservation of Herpetofauna in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia
CONSERVATION OF HERPETOFAUNA IN BANTIMURUNG BULUSARAUNG NATIONAL PARK, SOUTH SULAWESI, INDONESIA Final Report 2008 By: M. Irfansyah Lubis, Wempy Endarwin, Septiantina D. Riendriasari, Suwardiansah, Adininggar U. Ul-Hasanah, Feri Irawan, Hadijah Aziz K., and Akmal Malawi Departemen Konservasi Sumberdaya Hutan Fakultas Kehutanan Institut Pertanian Bogor Bogor Indonesia 16000 Tel : +62 – 251 – 621 947 Fax: +62 – 251 – 621 947 Email: [email protected] (team leader) Conservation of Herpetofauna in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia Executive Summary Sulawesi is an island with complex geological and geographical history, thus resulting in a complex array in biodiversity. Bantimurung Bulusaraung National Park (BabulNP) was gazetted in 2004 to protect the region’s biodiversity and karst ecosystem. However, the park’s herpetofauna is almost unknown. This project consists of three programs: herpetofauna survey in BabulNP, herpetofauna conservation education to local schools, and herpetofauna training for locals and was conducted from July to September 2007. Based on the survey conducted in six sites in the park, we recorded 12 amphibian and 25 reptile species. Five of those species (Bufo celebensis, Rana celebensis, Rhacophorus monticola, Sphenomorphus tropidonotus, and Calamaria muelleri) are endemic to Sulawesi. Two species of the genus Oreophryne are still unidentified. We visited six schools around the park for our herpetofauna conservation education program. The Herpetofauna Observation Training was held over four days with 17 participants from BabulNP staff, local NGOs, school teachers, and Hasanuddin University students. i Conservation of Herpetofauna in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia Acknowledgements This project would not have been possible without the contribution of many persons. We would like to express our gratitude to BP Conservation Leadership Programme for providing funding. -

ANIFIBIOS Y REPTILES De La Parroquia Urbana Lumbaquí 1 Manuel R
Cantón Gonzalo Pizarro, Sucumbíos, Ecuador ANIFIBIOS y REPTILES de la Parroquia Urbana Lumbaquí 1 Manuel R. Dueñas1 & Lesly Báez E.2 1Instituto Nacional de Biodiversidad (INABIO), 2Universidad Central del Ecuador, Facultad de Ciencias Biológicas y Ambientales. Fotografías: Manuel R. Dueñas (MRD), Lesly Báez E (LBE), Sebastián Valverde (SV), Juan Carlos Sánchez-Nivicela (JCSN), Santiago Varela Largo (SVL). Leyenda: (Juv): juvenil; (Sub): subadulto; (Met): metamórfico; (d): vista dorsal; (v): vista ventral; (l): vista lateral ingle; ♂: macho; ♀: hembra. Agradecimientos: Cecilia Tobar, Jorge H. Valencia, Mario H. Yánez-Muñoz, Juan Carlos Sánchez-Nivicela, Daniela Franco-Mena, Sebastián Valverde, Santiago Varela Largo, Luis Tipantiza- Tuguminago, Michelle Vela, Marco Benítez, Rosa Benítez.© Producido por Manuel R. Dueñas: [email protected] con el apoyo de Fundación EcoCiencia, Fundación Herpetológica Gustavo Orcés (FHGO), Instituto Nacional de Biodiversidad (INABIO). © Field Museum (2021) CC BY-NC 4.0. /RVWUDEDMRVFRQHVWDOLFHQFLDVRQOLEUHVGHXVDUFRPSDUWLUUHPH]FODUFRQDWULEXFLyQSHURQRSHUPLWHQ HOXVRFRPHUFLDOGHOWUDEDMRRULJLQDO [fieldguides.fieldmuseum.org] [1308] versión 1 3/2021 1 Allobates femoralis (Juv) 2 Allobates femoralis 3 Rhinella dapsilis 4 Rhinella margaritifera (Juv) AROMOBATIDAE AROMOBATIDAE BUFONIDAE BUFONIDAE (SV) (SV) (MRD) (MRD) 5 Rhinella margaritifera (Juv) 6 Rhinella margaritifera 7 Rhinella marina (Juv) 8 Rhinella marina BUFONIDAE BUFONIDAE BUFONIDAE BUFONIDAE (MRD) (MRD) (MRD) (MRD) 9 Teratohyla midas 10 Teratohyla -

Amphibia: Anura: Aromobatidae:Mannophryne
Artículo original / Original article HERPETOTROPICOS 2006 Vol. 3(1):51-57 Copyright © 2007 Univ. Los Andes VARGAS GALARCE and LA MARCA - A NEW SPECIES OF COLLAREDPrinted FROG in Venezuela. All rights reserved51 ISSN 1690-7930 A NEW SPECIES OF COLLARED FROG (AMPHIBIA: ANURA: AROMOBATIDAE: MANNOPHRYNE) FROM THE ANDES OF TRUJILLO STATE, VENEZUELA JÉSSICA Y. VARGAS GALARCE1,2 AND ENRIQUE LA MARCA2,3 1 Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela. 2 Laboratorio de Biogeografía, Escuela de Geografía, Facultad de Ciencias Forestales y Ambientales, Universidad de Los Andes, Mérida, Venezuela. Abstract: We describe a new species of Mannophryne frog, coming from Trujillo State, in the Venezuelan Andes, which constitutes the first species of the genus described from that political unit. This new taxon is distinguished from its congeners by the following combination of characters: small size (SVL males 20.1-23.1 mm, females 23.3-27.5 mm); tympanum about ½ the horizontal length of eye; first finger almost equal, or slightly shorter than second; fingers with thick lateral fringes; tarsal fold conspicuous; foot-web formula: I1.0–0.5II1.0–1.0III1.5–0.5IV0.5–2.0V; toes with lateral flaps; short oblique pale inguinal band; collar moderately wide, solid, with small pale blotches; ventrolateral band absent. We provide data on coloration, in life and in preservative, of specimens of the type series, as well as a description of the larvae and ecological data for the species. Key words: Mannophryne, Amphibia, Anura, Venezuela, Andes, taxonomy, natural history, conservation. Resumen: J.Y. Vargas Galarce y E. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Anura: Aromobatidae)
bioRxiv preprint doi: https://doi.org/10.1101/771287; this version posted September 16, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Sexual dichromatism in the neotropical genus Mannophryne (Anura: Aromobatidae) 2 Mark S. Greener1*, Emily Hutton2, Christopher J. Pollock3, Annabeth Wilson2, Chun Yin Lam2, Mohsen 3 Nokhbatolfoghahai 2, Michael J. Jowers4, and J. Roger Downie2 4 1Department of Pathology, Bacteriology and Avian Diseases, Faculty of Veterinary Medicine, Ghent 5 University, B-9820 Merelbeke, Belgium. 6 2 School of Life Sciences, Graham Kerr Building, University of Glasgow, Glasgow G12 8QQ, UK. 7 3 School of Biology, Faculty of Biological sciences, University of Leeds, Leeds LS2 9JT, UK. 8 4 CIBIO/InBIO (Centro de Investigacao em Biodiversidade e Recursos Genticos), Universide do Porto , 9 Vairao 4485-661, Portugal. 10 *[email protected] 11 ABSTRACT 12 Recent reviews on sexual dichromatism in frogs included Mannophryne trinitatis as the only example 13 they could find of dynamic dichromatism (males turn black when calling) within the family 14 Aromobatidae and found no example of ontogenetic dichromatism in this group. We demonstrate 15 ontogenetic dichromatism in M. trinitatis by rearing post-metamorphic froglets to near maturity: the 16 throats of all individuals started as grey coloured; at around seven weeks, the throat became pale 17 yellow in some, and more strongly yellow as development proceeded; the throats of adults are grey 18 in males and variably bright yellow in females, backed by a dark collar. -
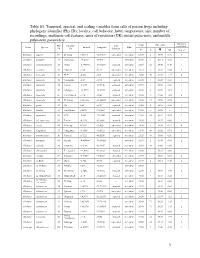
1 Table S1. Temporal, Spectral, and Scaling Variables from Calls Of
Table S1. Temporal, spectral, and scaling variables from calls of poison frogs including phylogeny identifier (Phy ID), locality, call behavior, habit, temperature, size, number of recordings, multinote call features, units of repetition (UR), initial pulse-note, and middle pulse-note parameters. Analyzed Phy Locality Call Temp SVL (mm) Genus Species Latitude Longitude Habit recordings ID ID Behavior °C N ! SD N of ♂ Allobates algorei 60 El Tama 7.65375 -72.19137 concealed terrestrial 23.50 8 18.90 0.70 3 Allobates brunneus 37 Guimaraes -15.2667 -55.5311 -- terrestrial 26.50 1 16.13 0.00 1 Allobates caeruleodactylus 48 Borba -4.398593 -59.60251 exposed terrestrial 25.60 12 15.50 0.40 1 Allobates crombiei 52 Altamira -3.65 -52.38 concealed terrestrial 24.10 2 18.10 0.04 2 Allobates femoralis 43 ECY -0.633 -76.5 concealed terrestrial 25.60 20 23.58 1.27 6 Allobates femoralis 46 Porongaba -8.67 -72.78 exposed terrestrial 25.00 1 25.38 0.00 1 Allobates femoralis 44 Leticia -4.2153 -69.9406 exposed terrestrial 25.50 1 20.90 0.00 1 Allobates femoralis 40 Albergue -12.8773 -71.3865 exposed terrestrial 26.00 6 21.98 2.18 1 Allobates femoralis 41 CAmazonico -12.6 -70.08 exposed terrestrial 26.00 12 22.43 1.06 4 Allobates femoralis 45 El Palmar 8.333333 -61.66667 concealed terrestrial 24.00 27 25.50 0.76 1 Allobates granti 49 FG 3.62 -53.17 exposed terrestrial 24.60 8 16.15 0.55 1 Allobates humilis 59 San Ramon 8.8678 -70.4861 concealed terrestrial 19.50 -- 21.80 -- 1 Allobates insperatus 54 ECY -0.633 -76.4005 exposed terrestrial 24.60 18 16.64 0.93 7 Allobates aff.