Transoceanic Dispersal and Plate Tectonics Shaped
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Aspects About Supella Longipalpa (Blattaria: Blattellidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Asian Pac J Trop Biomed 2016; 6(12): 1065–1075 1065 HOSTED BY Contents lists available at ScienceDirect Asian Pacific Journal of Tropical Biomedicine journal homepage: www.elsevier.com/locate/apjtb Review article http://dx.doi.org/10.1016/j.apjtb.2016.08.017 New aspects about Supella longipalpa (Blattaria: Blattellidae) Hassan Nasirian* Department of Medical Entomology and Vector Control, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran ARTICLE INFO ABSTRACT Article history: The brown-banded cockroach, Supella longipalpa (Blattaria: Blattellidae) (S. longipalpa), Received 16 Jun 2015 recently has infested the buildings and hospitals in wide areas of Iran, and this review was Received in revised form 3 Jul 2015, prepared to identify current knowledge and knowledge gaps about the brown-banded 2nd revised form 7 Jun, 3rd revised cockroach. Scientific reports and peer-reviewed papers concerning S. longipalpa and form 18 Jul 2016 relevant topics were collected and synthesized with the objective of learning more about Accepted 10 Aug 2016 health-related impacts and possible management of S. longipalpa in Iran. Like the Available online 15 Oct 2016 German cockroach, the brown-banded cockroach is a known vector for food-borne dis- eases and drug resistant bacteria, contaminated by infectious disease agents, involved in human intestinal parasites and is the intermediate host of Trichospirura leptostoma and Keywords: Moniliformis moniliformis. Because its habitat is widespread, distributed throughout Brown-banded cockroach different areas of homes and buildings, it is difficult to control. -

Cockroach Marion Copeland
Cockroach Marion Copeland Animal series Cockroach Animal Series editor: Jonathan Burt Already published Crow Boria Sax Tortoise Peter Young Ant Charlotte Sleigh Forthcoming Wolf Falcon Garry Marvin Helen Macdonald Bear Parrot Robert E. Bieder Paul Carter Horse Whale Sarah Wintle Joseph Roman Spider Rat Leslie Dick Jonathan Burt Dog Hare Susan McHugh Simon Carnell Snake Bee Drake Stutesman Claire Preston Oyster Rebecca Stott Cockroach Marion Copeland reaktion books Published by reaktion books ltd 79 Farringdon Road London ec1m 3ju, uk www.reaktionbooks.co.uk First published 2003 Copyright © Marion Copeland All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publishers. Printed and bound in Hong Kong British Library Cataloguing in Publication Data Copeland, Marion Cockroach. – (Animal) 1. Cockroaches 2. Animals and civilization I. Title 595.7’28 isbn 1 86189 192 x Contents Introduction 7 1 A Living Fossil 15 2 What’s in a Name? 44 3 Fellow Traveller 60 4 In the Mind of Man: Myth, Folklore and the Arts 79 5 Tales from the Underside 107 6 Robo-roach 130 7 The Golden Cockroach 148 Timeline 170 Appendix: ‘La Cucaracha’ 172 References 174 Bibliography 186 Associations 189 Websites 190 Acknowledgements 191 Photo Acknowledgements 193 Index 196 Two types of cockroach, from the first major work of American natural history, published in 1747. Introduction The cockroach could not have scuttled along, almost unchanged, for over three hundred million years – some two hundred and ninety-nine million before man evolved – unless it was doing something right. -

Bulletin Number / Numéro 2 Entomological Society of Canada Société D’Entomologie Du Canada June / Juin 2008
Volume 40 Bulletin Number / numéro 2 Entomological Society of Canada Société d’entomologie du Canada June / juin 2008 Published quarterly by the Entomological Society of Canada Publication trimestrielle par la Société d’entomologie du Canada ............................................................... .................................................................................................................................................................................................................................................................................................................................. .......................................................................... ........................................................................................................................................................................ ....................... ................................................................................. ................................................. List of contents / Table des matières Volume 40 (2), June / june 2008 Up front / Avant-propos ................................................................................................................49 Moth balls / Boules à mites .............................................................................................................51 Meeting announcements / Réunions futures ..................................................................................52 Dear Buggy / Cher Bibitte ..............................................................................................................53 -
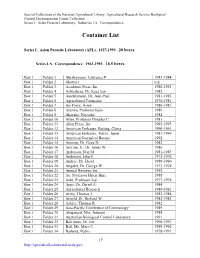
Container List
Special Collections of the National Agricultural Library: Agricultural Research Service Biological Control Documentation Center Collection Series I. Asian Parasite Laboratory. Subseries I.A. Correspondence. Container List Series I. Asian Parasite Laboratory (APL). 1927-1993. 20 boxes. Series I.A. Correspondence. 1963-1993. 16.5 boxes. Box 1 Folder 1 Abrahamson, Lawrence P. 1983-1984 Box 1 Folder 2 Abstract n.d. Box 1 Folder 3 Academic Press, Inc. 1986-1993 Box 1 Folder 4 Achterberg, Dr. Kees van 1982 Box 1 Folder 5 Aeschlimann, Dr. Jean-Paul 1981-1985 Box 1 Folder 6 Agricultural Counselor 1976-1981 Box 1 Folder 7 Air Force, Army 1980-1981 Box 1 Folder 8 Aizawa, Professor Keio 1986 Box 1 Folder 9 Akasaka, Naoyuki 1982 Box 1 Folder 10 Allen, Professor Douglas C. 1981 Box 1 Folder 11 Allen Press, Inc. 1992-1993 Box 1 Folder 12 American Embassy, Beijing, China 1990-1991 Box 1 Folder 13 American Embassy, Tokyo, Japan 1981-1990 Box 1 Folder 14 American Journal of Botany 1992 Box 1 Folder 15 Amman, Dr. Gene D. 1982 Box 1 Folder 16 Amrine, Jr., Dr. James W. 1986 Box 1 Folder 17 Anderson, Don M. 1981-1983 Box 1 Folder 18 Anderson, John F. 1975-1976 Box 1 Folder 19 Andres, Dr. Lloyd 1989-1990 Box 1 Folder 20 Angalet, Dr. George W. 1973-1978 Box 1 Folder 21 Annual Reviews Inc. 1992 Box 1 Folder 22 Ao, Professor Hsien-Bine 1988 Box 1 Folder 23 Aoki, Professor Joji 1977-1978 Box 1 Folder 24 Apps, Dr. Darrel A. 1984 Box 1 Folder 25 Agricultural Research 1980-1981 Box 1 Folder 26 Army, Thomas J. -

Running Speed and Food Intake of the Matrotrophic Viviparous Cockroach Diploptera Punctata (Blattodea: Blaberidae) During Gestation
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomologie heute Jahr/Year: 2014 Band/Volume: 26 Autor(en)/Author(s): Greven Hartmut, Floßdorf David, Köthe Janine, List Fabian, Zwanzig Nadine Artikel/Article: Running Speed and Food Intake of the Matrotrophic Viviparous Cockroach Diploptera punctata (Blattodea: Blaberidae) during Gestation. Laufgeschwindigkeit und Nahrungsaufnahme der matrotroph viviparen Schabe Diploptera punctata (Blattodea: Blaberidae) während der Trächtigkeit 53-72 Running speed and food intake of Diploptera punctata during gestation 53 Entomologie heute 26 (2014): 53-72 Running Speed and Food Intake of the Matrotrophic Viviparous Cockroach Diploptera punctata (Blattodea: Blaberidae) during Gestation Laufgeschwindigkeit und Nahrungsaufnahme der matrotroph viviparen Schabe Diploptera punctata (Blattodea: Blaberidae) während der Trächtigkeit HARTMUT GREVEN, DAVID FLOSSDORF, JANINE KÖTHE, FABIAN LIST & NADINE ZWANZIG Summary: Diploptera punctata is the only cockroach, which has been clearly characterized as matro- trophic viviparous. Our observations on courtship and mating generally confi rm the data from the literature. Courtship and mating correspond to type I (male offers himself under wing fl uttering, female mounts the male, nibbles on his tergal glands, dismounts, turns to achieve the fi nal mating position, i.e. abdomen to abdomen, heads in the opposite direction. We document photographic ally mating and courtship of fully sklerotized, sexually experienced males with teneral females imme- diately after the last moult, and with fully sclerotized females several hours after the fi nal moult. Effects of sexual dimorphism (females are larger than males) and pregnancy (females gain weight) became apparent from the running speed cockroaches reached, when disturbed. Males and females ran signifi cantly faster during daytime than at night, but males ran always faster than females. -

Long Rdna Amplicon Sequencing of Insect-Infecting Nephridiophagids
www.nature.com/scientificreports OPEN Long rDNA amplicon sequencing of insect‑infecting nephridiophagids reveals their afliation to the Chytridiomycota and a potential to switch between hosts Jürgen F. H. Strassert 1*, Christian Wurzbacher 2, Vincent Hervé 3, Taraha Antany1, Andreas Brune 3 & Renate Radek 1* Nephridiophagids are unicellular eukaryotes that parasitize the Malpighian tubules of numerous insects. Their life cycle comprises multinucleate vegetative plasmodia that divide into oligonucleate and uninucleate cells, and sporogonial plasmodia that form uninucleate spores. Nephridiophagids are poor in morphological characteristics, and although they have been tentatively identifed as early‑branching fungi based on the SSU rRNA gene sequences of three species, their exact position within the fungal tree of live remained unclear. In this study, we describe two new species of nephridiophagids (Nephridiophaga postici and Nephridiophaga javanicae) from cockroaches. Using long‑read sequencing of the nearly complete rDNA operon of numerous further species obtained from cockroaches and earwigs to improve the resolution of the phylogenetic analysis, we found a robust afliation of nephridiophagids with the Chytridiomycota—a group of zoosporic fungi that comprises parasites of diverse host taxa, such as microphytes, plants, and amphibians. The presence of the same nephridiophagid species in two only distantly related cockroaches indicates that their host specifcity is not as strict as generally assumed. Insects are the most diverse group of all animals. So far, about one million species have been described and recent estimates for extant species range from 2.6 to 7.8 million1,2. Tey are globally distributed and impact human life at numerous levels. In agriculture, for instance, insects play a major role as both pollinators (e.g., honey bees) and pests that feed on crops (e.g., grasshoppers). -

Cockroach Control Manual
COCKROACHCOCKROACH CONTROLCONTROL MANUALMANUAL (Photo by J. Kalisch) Barb Ogg, Extension Educator, Lancaster County Clyde Ogg, Extension Educator, Pesticide Safety Education Program Dennis Ferraro, Extension Educator, Douglas & Sarpy Counties Extension is a Division of the Institute of Agriculture and Natural Resources at the University of Nebraska–Lincoln cooperating with the Counties and the United States Department of Agriculture. ® University of Nebraska–Lincoln Extension’s educational programs abide with the nondiscrimination policies of the University of Nebraska–Lincoln and the United States Department of Agriculture. Table of Contents 1 Chapter 1: Introduction 5 Chapter 2: Know Your Enemy 9 Chapter 3: Cockroach Biology 15 Chapter 4: Locate Problem Areas 23 Chapter 5: Primary Control Strategies: Modify Resources 31 Chapter 6: Low-Risk Control Strategies 37 Chapter 7: Insecticide Basics 45 Chapter 8: Insecticides and Your Health 53 Chapter 9: Insecticide Applications 59 Chapter 10: Putting a Management Plan Together i Cockroach Control Manual Preface It has been more than 10 years since the first edition of the Cockroach Control Manual was completed. While the basic steps for effective and safe cockroach control are still the same, there are more types of control products available than there were 10 years ago. This means you have even more choices in your arsenal to help fight roaches. The Cockroach Control Manual is a practical reference for persons who have had little or no training in insect identification, biology or control methods. We know most people want low toxic methods used inside their homes so we are emphasizing low-risk strategies even more than in the original edition. -

A Dichotomous Key for the Identification of the Cockroach Fauna (Insecta: Blattaria) of Florida
Species Identification - Cockroaches of Florida 1 A Dichotomous Key for the Identification of the Cockroach fauna (Insecta: Blattaria) of Florida Insect Classification Exercise Department of Entomology and Nematology University of Florida, Gainesville 32611 Abstract: Students used available literature and specimens to produce a dichotomous key to species of cockroaches recorded from Florida. This exercise introduced students to techniques used in studying a group of insects, in this case Blattaria, to produce a regional species key. Producing a guide to a group of insects as a class exercise has proven useful both as a teaching tool and as a method to generate information for the public. Key Words: Blattaria, Florida, Blatta, Eurycotis, Periplaneta, Arenivaga, Compsodes, Holocompsa, Myrmecoblatta, Blatella, Cariblatta, Chorisoneura, Euthlastoblatta, Ischnoptera,Latiblatta, Neoblatella, Parcoblatta, Plectoptera, Supella, Symploce,Blaberus, Epilampra, Hemiblabera, Nauphoeta, Panchlora, Phoetalia, Pycnoscelis, Rhyparobia, distributions, systematics, education, teaching, techniques. Identification of cockroaches is limited here to adults. A major source of confusion is the recogni- tion of adults from nymphs (Figs. 1, 2). There are subjective differences, as well as morphological differences. Immature cockroaches are known as nymphs. Nymphs closely resemble adults except nymphs are generally smaller and lack wings and genital openings or copulatory appendages at the tip of their abdomen. Many species, however, have wingless adult females. Nymphs of these may be recognized by their shorter, relatively broad cerci and lack of external genitalia. Male cockroaches possess styli in addition to paired cerci. Styli arise from the subgenital plate and are generally con- spicuous, but may also be reduced in some species. Styli are absent in adult females and nymphs. -

Towards Classical Biological Control of Leek Moth
____________________________________________________________________________ Ateyyat This project seeks to provide greater coherence for the biocontrol knowledge system for regulators and researchers; create an open access information source for biocontrol re- search of agricultural pests in California, which will stimulate greater international knowl- edge sharing about agricultural pests in Mediterranean climates; and facilitate the exchange of information through a cyberinfrastructure among government regulators, and biocontrol entomologists and practitioners. It seeks broader impacts through: the uploading of previ- ously unavailable data being made openly accessible; the stimulation of greater interaction between the biological control regulation, research, and practitioner community in selected Mediterranean regions; the provision of more coherent and useful information to enhance regulatory decisions by public agency scientists; a partnership with the IOBC to facilitate international data sharing; and progress toward the ultimate goal of increasing the viability of biocontrol as a reduced risk pest control strategy. No Designated Session Theme BIOLOGY OF CIRROSPILUS INGENUUS GAHAN (HYMENOPTERA: EULOPHIDAE), AN ECTOPARASITOID OF THE CITRUS LEAFMINER, PHYLLOCNISTIS CITRELLA STAINTON (LEPIDOPTERA: GRACILLARIIDAE) ON LEMON 99 Mazen A. ATEYYAT Al-Shoubak University College, Al-Balqa’ Applied University, P.O. Box (5), Postal code 71911, Al-Shawbak, Jordan [email protected] The citrus leafminer (CLM), Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) in- vaded the Jordan Valley in 1994 and was able to spread throughout Jordan within a few months of its arrival. It was the most common parasitoid from 1997 to 1999 in the Jordan Valley. An increase in the activity of C. ingenuus was observed in autumn and the highest number of emerged C. ingenuus adults was in November 1999. -

Phylogeny of Endopterygote Insects, the Most Successful Lineage of Living Organisms*
REVIEW Eur. J. Entomol. 96: 237-253, 1999 ISSN 1210-5759 Phylogeny of endopterygote insects, the most successful lineage of living organisms* N iels P. KRISTENSEN Zoological Museum, University of Copenhagen, Universitetsparken 15, DK-2100 Copenhagen 0, Denmark; e-mail: [email protected] Key words. Insecta, Endopterygota, Holometabola, phylogeny, diversification modes, Megaloptera, Raphidioptera, Neuroptera, Coleóptera, Strepsiptera, Díptera, Mecoptera, Siphonaptera, Trichoptera, Lepidoptera, Hymenoptera Abstract. The monophyly of the Endopterygota is supported primarily by the specialized larva without external wing buds and with degradable eyes, as well as by the quiescence of the last immature (pupal) stage; a specialized morphology of the latter is not an en dopterygote groundplan trait. There is weak support for the basal endopterygote splitting event being between a Neuropterida + Co leóptera clade and a Mecopterida + Hymenoptera clade; a fully sclerotized sitophore plate in the adult is a newly recognized possible groundplan autapomorphy of the latter. The molecular evidence for a Strepsiptera + Díptera clade is differently interpreted by advo cates of parsimony and maximum likelihood analyses of sequence data, and the morphological evidence for the monophyly of this clade is ambiguous. The basal diversification patterns within the principal endopterygote clades (“orders”) are succinctly reviewed. The truly species-rich clades are almost consistently quite subordinate. The identification of “key innovations” promoting evolution -

Savannah Guides Communicator, January 2011
PO Box 2312 Cairns QLD 4870 Phone: 0408 772 513 E-mail: [email protected] Web site: www.savannah-guides.com.au January 2011 Inside this issue: Words from our President - Ben Humphries Page 2 - New Board members Congratulations everyone on another successful year in the tourism industry. Varying reports from Cairns suggest a Page 3 - Drive North Qld! quiet year for tourism, similar reports from Kakadu, with visitor numbers down on previous years. Despite this quiet period, Savannah Guides has continued to lead the field in ‘Protecting and Interpreting the Outback’. Page 4 - Andrew Underground Highlights from 2010 include our Savannah Guide Schools, held at Undara in March and in the Tablelands in October. These Schools are fantastic opportunities to catch up with mates, develop networks and new skills, and learn more Page 5 - Tigers in the Bush from local experts on the region. A lot of work goes into planning and implementing a School and a huge thanks to all those people who were involved and committed their time to the success of our organisation. Thank you to Page 6 - Dinosaurs to Dunnarts- Andrew and his Team from Undara for hosting a terrific School in Outback Queensland in March. Biodiversity Program Congratulations to founding members who were bestowed with Life Membership Awards: Bruce Butler, Barry Kubala, Tom Warnes and Gerry Collins. John Courtenay was bestowed with the Lifetime of Leadership Award. Many Page 7 - Beautiful Cockroaches good memories were shared and we all witnessed the power of the passion within Savannah Guides as the emotions ran high. -

Cockroaches Introduction Members of the Order Blattodea, There Are About 4,500 Species of Cockroach, Which Live in a Wide Range of Environments Around the World
The following sample is taken from Breeding Insects as Feeder Food published as both an ebook and in hardcopy on Amazon. The images have been removed from this sample but the formatting is retained. Cockroaches Introduction Members of the order Blattodea, there are about 4,500 species of cockroach, which live in a wide range of environments around the world. Although considered pests associated with disease, there are only about 30 species that are commonly found in proximity with humans and, of these, only four are considered serious pests. Cockroaches are mainly nocturnal and will normally try to avoid light. Although most species prefer warm climates, cockroaches are among the hardiest insects and can survive extremes at both end of the temperature scale. Some species are capable of remaining active for a month without food and are able to survive on very limited sustenance. They are omnivorous, feeding on organic materials, including decaying plant matter, but they will also eat dead insects and animals. Compared to crickets, cockroaches have some advantages as feeder insects. They are unlikely to attack a small chameleon in the same way that a cricket might. They are longer lived and are hardier. Most are easier to breed than crickets and they make no noise! Unfortunately, despite these advantages, they are not as readily accepted by some animals as crickets are. Cockroaches suffer from a perception they harbour disease and that they smell. The species shown below will not do so if kept in clean conditions and fed correctly. For mantis, cockroaches can be a better choice than crickets.