Report 1978 Cold Spring Harbor Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RANDY SCHEKMAN Department of Molecular and Cell Biology, Howard Hughes Medical Institute, University of California, Berkeley, USA
GENES AND PROTEINS THAT CONTROL THE SECRETORY PATHWAY Nobel Lecture, 7 December 2013 by RANDY SCHEKMAN Department of Molecular and Cell Biology, Howard Hughes Medical Institute, University of California, Berkeley, USA. Introduction George Palade shared the 1974 Nobel Prize with Albert Claude and Christian de Duve for their pioneering work in the characterization of organelles interrelated by the process of secretion in mammalian cells and tissues. These three scholars established the modern field of cell biology and the tools of cell fractionation and thin section transmission electron microscopy. It was Palade’s genius in particular that revealed the organization of the secretory pathway. He discovered the ribosome and showed that it was poised on the surface of the endoplasmic reticulum (ER) where it engaged in the vectorial translocation of newly synthesized secretory polypeptides (1). And in a most elegant and technically challenging investigation, his group employed radioactive amino acids in a pulse-chase regimen to show by autoradiograpic exposure of thin sections on a photographic emulsion that secretory proteins progress in sequence from the ER through the Golgi apparatus into secretory granules, which then discharge their cargo by membrane fusion at the cell surface (1). He documented the role of vesicles as carriers of cargo between compartments and he formulated the hypothesis that membranes template their own production rather than form by a process of de novo biogenesis (1). As a university student I was ignorant of the important developments in cell biology; however, I learned of Palade’s work during my first year of graduate school in the Stanford biochemistry department. -

ANNUAL REVIEW 1 October 2005–30 September
WELLCOME TRUST ANNUAL REVIEW 1 October 2005–30 September 2006 ANNUAL REVIEW 2006 The Wellcome Trust is the largest charity in the UK and the second largest medical research charity in the world. It funds innovative biomedical research, in the UK and internationally, spending around £500 million each year to support the brightest scientists with the best ideas. The Wellcome Trust supports public debate about biomedical research and its impact on health and wellbeing. www.wellcome.ac.uk THE WELLCOME TRUST The Wellcome Trust is the largest charity in the UK and the second largest medical research charity in the world. 123 CONTENTS BOARD OF GOVERNORS 2 Director’s statement William Castell 4 Advancing knowledge Chairman 16 Using knowledge Martin Bobrow Deputy Chairman 24 Engaging society Adrian Bird 30 Developing people Leszek Borysiewicz 36 Facilitating research Patricia Hodgson 40 Developing our organisation Richard Hynes 41 Wellcome Trust 2005/06 Ronald Plasterk 42 Financial summary 2005/06 Alastair Ross Goobey 44 Funding developments 2005/06 Peter Smith 46 Streams funding 2005/06 Jean Thomas 48 Technology Transfer Edward Walker-Arnott 49 Wellcome Trust Genome Campus As at January 2007 50 Public Engagement 51 Library and information resources 52 Advisory committees Images 1 Surface of the gut. 3 Zebrafish. 5 Cells in a developing This Annual Review covers the 2 Young children in 4 A scene from Y fruit fly. Wellcome Trust’s financial year, from Kenya. Touring’s Every Breath. 6 Data management at the Sanger Institute. 1 October 2005 to 30 September 2006. CONTENTS 1 45 6 EXECUTIVE BOARD MAKING A DIFFERENCE Developing people: To foster a Mark Walport The Wellcome Trust’s mission is research community and individual Director to foster and promote research with researchers who can contribute to the advancement and use of knowledge Ted Bianco the aim of improving human and Director of Technology Transfer animal health. -

Attachment 1
Appendix 1 Chemico-Biological Interactions 301 (2019) 2–5 Contents lists available at ScienceDirect Chemico-Biological Interactions journal homepage: www.elsevier.com/locate/chembioint An examination of the linear no-threshold hypothesis of cancer risk T assessment: Introduction to a series of reviews documenting the lack of biological plausibility of LNT R. Goldena,*, J. Busb, E. Calabresec a ToxLogic, Gaithersburg, MD, USA b Exponent, Midland, MI, USA c University of Massachusetts, Amherst, MA, USA The linear no-threshold (LNT) single-hit dose response model for evolution and the prominence of co-author Gilbert Lewis, who would be mutagenicity and carcinogenicity has dominated the field of regulatory nominated for the Nobel Prize some 42 times, this idea generated much risk assessment of carcinogenic agents since 1956 for radiation [8] and heat but little light. This hypothesis was soon found to be unable to 1977 for chemicals [11]. The fundamental biological assumptions upon account for spontaneous mutation rates, underestimating such events which the LNT model relied at its early adoption at best reflected a by a factor of greater than 1000-fold [19]. primitive understanding of key biological processes controlling muta- Despite this rather inauspicious start for the LNT model, Muller tion and development of cancer. However, breakthrough advancements would rescue it from obscurity, giving it vast public health and medical contributed by modern molecular biology over the last several decades implications, even proclaiming it a scientific principle by calling it the have provided experimental tools and evidence challenging the LNT Proportionality Rule [20]. While initially conceived as a driving force model for use in risk assessment of radiation or chemicals. -

Vol 9 No 1 Spring
A PUBLICATION OF THE AMERICAN SOCIETY FOR MATRIX BIOLOGY SPRING 2010, VOLUME 9, NO. 1 President’s Letter Expanding the Society’s Value to You and Your Role in the Society Since its inception in 2001, the principal function of the ASMB has been to organize the OFFICERS biennial meeting. The value of this meeting to our members cannot be understated. The ASMB meeting has emerged as the matrix-centric President: meeting in North America, and it provides an William Parks (2010) open venue for students, postdocs, fellows, and junior faculty to present their work and to inter- act with established investigators. Speaking for Vice Pres/President Elect myself, I very much look forward to the ASMB Jean Schwarzbauer (2010) meeting, not only because I get to see many Bill Parks friends, but also to hear a lot of incredibly good science. This year’s meeting will be no excep- Past President: tion and promises to be truly outstanding. I applaud Jean Schwarzbauer and the Renato Iozzo (2010) rest of the Program Committee for putting together an exciting meeting loaded with interesting topics and great speakers (please check out the program here). By organizing the meeting, ASMB provides value to you, the membership, but I Secretary/Treasurer think we–the Society–should always be looking to do more; that is, to provide more Joanne Murphy-Ullrich (2011) bang for your dues buck. For this year’s meeting, we have expanded the Travel Awards that will be given to trainees in recognition of outstanding research, and we recently established merit-based Minority Scholarships that will be awarded to eli- Council Members gible students and postdocs. -

A Short History of DNA Technology 1865 - Gregor Mendel the Father of Genetics
A Short History of DNA Technology 1865 - Gregor Mendel The Father of Genetics The Augustinian monastery in old Brno, Moravia 1865 - Gregor Mendel • Law of Segregation • Law of Independent Assortment • Law of Dominance 1865 1915 - T.H. Morgan Genetics of Drosophila • Short generation time • Easy to maintain • Only 4 pairs of chromosomes 1865 1915 - T.H. Morgan •Genes located on chromosomes •Sex-linked inheritance wild type mutant •Gene linkage 0 •Recombination long aristae short aristae •Genetic mapping gray black body 48.5 body (cross-over maps) 57.5 red eyes cinnabar eyes 67.0 normal wings vestigial wings 104.5 red eyes brown eyes 1865 1928 - Frederick Griffith “Rough” colonies “Smooth” colonies Transformation of Streptococcus pneumoniae Living Living Heat killed Heat killed S cells mixed S cells R cells S cells with living R cells capsule Living S cells in blood Bacterial sample from dead mouse Strain Injection Results 1865 Beadle & Tatum - 1941 One Gene - One Enzyme Hypothesis Neurospora crassa Ascus Ascospores placed X-rays Fruiting on complete body medium All grow Minimal + amino acids No growth Minimal Minimal + vitamins in mutants Fragments placed on minimal medium Minimal plus: Mutant deficient in enzyme that synthesizes arginine Cys Glu Arg Lys His 1865 Beadle & Tatum - 1941 Gene A Gene B Gene C Minimal Medium + Citruline + Arginine + Ornithine Wild type PrecursorEnz A OrnithineEnz B CitrulineEnz C Arginine Metabolic block Class I Precursor OrnithineEnz B CitrulineEnz C Arginine Mutants Class II Mutants PrecursorEnz A Ornithine -

Control of Molecular Motor Motility in Electrical Devices
Control of Molecular Motor Motility in Electrical Devices Thesis submitted in accordance with the requirements of The University of Liverpool for the degree of Doctor in Philosophy By Laurence Charles Ramsey Department of Electrical Engineering & Electronics April 2014 i Abstract In the last decade there has been increased interest in the study of molecular motors. Motor proteins in particular have gained a large following due to their high efficiency of force generation and the ability to incorporate the motors into linear device designs. Much of the recent research centres on using these protein systems to transport cargo around the surface of a device. The studies carried out in this thesis aim to investigate the use of molecular motors in lab- on-a-chip devices. Two distinct motor protein systems are used to show the viability of utilising these nanoscale machines as a highly specific and controllable method of transporting molecules around the surface of a lab-on-a-chip device. Improved reaction kinetics and increased detection sensitivity are just two advantages that could be achieved if a motor protein system could be incorporated and appropriately controlled within a device such as an immunoassay or microarray technologies. The first study focuses on the motor protein system Kinesin. This highly processive motor is able to propel microtubules across a surface and has shown promise as an in vitro nanoscale transport system. A novel device design is presented where the motility of microtubules is controlled using the combination of a structured surface and a thermoresponsive polymer. Both topographic confinement of the motility and the creation of localised ‘gates’ are used to show a method for the control and guidance of microtubules. -

EMBC Annual Report 2007
EMBO | EMBC annual report 2007 EUROPEAN MOLECULAR BIOLOGY ORGANIZATION | EUROPEAN MOLECULAR BIOLOGY CONFERENCE EMBO | EMBC table of contents introduction preface by Hermann Bujard, EMBO 4 preface by Tim Hunt and Christiane Nüsslein-Volhard, EMBO Council 6 preface by Marja Makarow and Isabella Beretta, EMBC 7 past & present timeline 10 brief history 11 EMBO | EMBC | EMBL aims 12 EMBO actions 2007 15 EMBC actions 2007 17 EMBO & EMBC programmes and activities fellowship programme 20 courses & workshops programme 21 young investigator programme 22 installation grants 23 science & society programme 24 electronic information programme 25 EMBO activities The EMBO Journal 28 EMBO reports 29 Molecular Systems Biology 30 journal subject categories 31 national science reviews 32 women in science 33 gold medal 34 award for communication in the life sciences 35 plenary lectures 36 communications 37 European Life Sciences Forum (ELSF) 38 ➔ 2 table of contents appendix EMBC delegates and advisers 42 EMBC scale of contributions 49 EMBO council members 2007 50 EMBO committee members & auditors 2007 51 EMBO council members 2008 52 EMBO committee members & auditors 2008 53 EMBO members elected in 2007 54 advisory editorial boards & senior editors 2007 64 long-term fellowship awards 2007 66 long-term fellowships: statistics 82 long-term fellowships 2007: geographical distribution 84 short-term fellowship awards 2007 86 short-term fellowships: statistics 104 short-term fellowships 2007: geographical distribution 106 young investigators 2007 108 installation -

Hx of Derm Final
DERMATOLOGY AT WASHINGTON UNIVERSITY THE TRADITION The tradition of Dermatology at Washington University School of Medicine can be best traced back to the earliest history of the Siteman Cancer Center in the late 1800s. After a tornado destroyed the old City Hospital in 1896, cancer patients were turned away from the emergency quarters that were established in the House of the Good Shephard. In 1905, in an effort to provide free cancer care to the poor, the St. Louis Skin and Cancer Hospital was founded in the old Tuholske Hospital. A few years later, a wealthy St. Louis Barnard Free Skin and Cancer Hospital businessman, George D. Barnard, financed the Barnard Circa ~ 1940. Located on Washington and Free Skin and Cancer Hospital for $130, 000 Theresa St., St, Louis MO. http://www.siteman.wustl.edu/internal.aspx?id=41. In the earliest days of Dermatology in St. Louis, over 50 physicians were trained as dermatologists through the Barnard Free Skin and Cancer Hospital. Early studies conducted at Barnard included work on fungi (Dr. Morris Moore), the epidermis (Dr. E.V. Cowdy) and cancer [1]. This initially free standing 44-bed hospital was later integrated into the Washington University School of Medicine in 1952. The current Barnard Hospital was erected in the Barnes complex in 1954. THE EARLY YEARS In the earliest years of Dermatology associated with Washington University, there were times when there was one “acting head”, and at others, two professors shared the responsibility. The first mention of a practicing dermatologist officially affiliated with Washington University was in the early 1900s. -

Moore Noller
2002 Ada Doisy Lectures Ada Doisy Lecturers 2003 in BIOCHEMISTRY Sponsored by the Department of Biochemistry • University of Illinois at Urbana-Champaign Dr. Peter B. 1970-71 Charles Huggins* and Elwood V. Jensen A76 1972-73 Paul Berg* and Walter Gilbert* Moore 1973-74 Saul Roseman and Bruce Ames Department of Molecular carbonyl Biophysics & Biochemistry Phe 1974-75 Arthur Kornberg* and Osamu Hayaishi Yale University C75 1976-77 Luis F. Leloir* New Haven, Connecticutt 1977-78 Albert L. Lehninger and Efraim Racker 2' OH attacking 1978-79 Donald D. Brown and Herbert Boyer amino N3 Tyr 1979-80 Charles Yanofsky A76 4:00 p.m. A2486 1980-81 Leroy E. Hood Thursday, May 1, 2003 (2491) 1983-84 Joseph L. Goldstein* and Michael S. Brown* Medical Sciences Auditorium 1984-85 Joan Steitz and Phillip Sharp* Structure and Function in 1985-86 Stephen J. Benkovic and Jeremy R. Knowles the Large Ribosomal Subunit 1986-87 Tom Maniatis and Mark Ptashne 1988-89 J. Michael Bishop* and Harold E. Varmus* 1989-90 Kurt Wüthrich Dr. Harry F. 1990-91 Edmond H. Fischer* and Edwin G. Krebs* 1993-94 Bert W. O’Malley Noller 1994-95 Earl W. Davie and John W. Suttie Director, Center for Molecular Biology of RNA 1995-96 Richard J. Roberts* University of California, Santa Cruz 1996-97 Ronald M. Evans Santa Cruz, California 1998-99 Elizabeth H. Blackburn 1999-2000 Carl R. Woese and Norman R. Pace 2000-01 Willem P. C. Stemmer and Ronald W. Davis 2001-02 Janos K. Lanyi and Sir John E. Walker* 12:00 noon 2002-03 Peter B. -
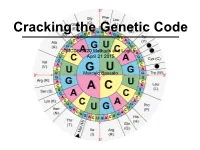
MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo
Cracking the Genetic Code MCDB 5220 Methods and Logics April 21 2015 Marcelo Bassalo The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) Frederick Griffith The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) Oswald Avery Alfred Hershey Martha Chase The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) Linus Carl Pauling The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) James Watson and Francis Crick The DNA Saga… so far Important contributions for cracking the genetic code: • The “transforming principle” (1928) • The nature of the transforming principle: DNA (1944 - 1952) • X-ray diffraction and the structure of proteins (1951) • The structure of DNA (1953) How is DNA (4 nucleotides) the genetic material while proteins (20 amino acids) are the building blocks? ? DNA Protein ? The Coding Craze ? DNA Protein What was already known? • DNA resides inside the nucleus - DNA is not the carrier • Protein synthesis occur in the cytoplasm through ribosomes {• Only RNA is associated with ribosomes (no DNA) - rRNA is not the carrier { • Ribosomal RNA (rRNA) was a homogeneous population The “messenger RNA” hypothesis François Jacob Jacques Monod The Coding Craze ? DNA RNA Protein RNA Tie Club Table from Wikipedia The Coding Craze Who won the race Marshall Nirenberg J. -

Identification of the C-Terminal Activator Domain in Yeast Heat Shock Factor: Independent Control of Transient and Sustained Transcriptional Activity
The EMBO Journal vol.12 no.13 pp.5007-5018, 1993 Identification of the C-terminal activator domain in yeast heat shock factor: independent control of transient and sustained transcriptional activity Yuqing Chen1, Nickolai A.Barlev2,3, Introduction Ole Westergaard and Bent K.Jakobsen4 In response to hyperthermia, and certain other forms of Department of Molecular Biology, University of Aarhus, C.F.Mollers stress, cells transiently increase transcription from a small Alle, Building 130, DK-8000 Aarhus C, Denmark group of genes that encode a characteristic set of proteins, lPresent address: Department of Zoology, University of Western the heat shock proteins. The heat shock proteins exercise Ontario, London, Canada protective functions in the cell during stress, for instance 2Present address: Laboratory of Structural Genome Organization, Institute of Cytology, Tihkoretsky Avenue 4, 194064 St Petersburg, by renaturing or solubilizing denatured proteins (for reviews Russia see Lindquist, 1986; Pelham, 1986; Bienz and Pelham, 3Present address: Wistar Institute, Pennsylvania University, 1987). 3601 Spruce Street, Philadelphia, PA 19104-4268, USA Heat shock promoters contain a universal sequence 4Present address: Institute of Molecular Medicine, University of element that is necessary and sufficient for their Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK transcriptional activation (the heat shock element, HSE). This Communicated by H.R.B.Pelham was originally identified as a 14 bp sequence by deletion analysis of the Drosophila hsp7O promoter (Bienz and In yeast, heat shock factor (HSF) is a trimer that binds Pelham, 1982; Pelham, 1982; Pelham and Bienz, 1982). DNA constitutively but only supports high levels of Careful examination of the HSE has demonstrated that it can transcription upon heat shock. -

Humankind 2.0: the Technologies of the Future 6. Biotech
Humankind 2.0: The Technologies of the Future 6. Biotech Piero Scaruffi, 2017 See http://www.scaruffi.com/singular/human20.html for the full text of this discussion A brief History of Biotech 1953: Discovery of the structure of the DNA 2 A brief History of Biotech 1969: Jon Beckwith isolates a gene 1973: Stanley Cohen and Herbert Boyer create the first recombinant DNA organism 1974: Waclaw Szybalski coins the term "synthetic biology” 1975: Paul Berg organizes the Asilomar conference on recombinant DNA 3 A brief History of Biotech 1976: Genentech is founded 1977: Fred Sanger invents a method for rapid DNA sequencing and publishes the first full DNA genome of a living being Janet Rossant creates a chimera combining two mice species 1980: Genentech’s IPO, first biotech IPO 4 A brief History of Biotech 1982: The first biotech drug, Humulin, is approved for sale (Eli Lilly + Genentech) 1983: Kary Mullis invents the polymerase chain reaction (PCR) for copying genes 1986: Leroy Hood invents a way to automate gene sequencing 1986: Mario Capecchi performs gene editing on a mouse 1990: William French Anderson’s gene therapy 1990: First baby born via PGD (Alan Handyside’s lab) 5 A brief History of Biotech 1994: FlavrSavr Tomato 1994: Maria Jasin’s homing endonucleases for genome editing 1996: Srinivasan Chandrasegaran’s ZFN method for genome editing 1996: Ian Wilmut clones the first mammal, the sheep Dolly 1997: Dennis Lo detects fetal DNA in the mother’s blood 2000: George Davey Smith introduces Mendelian randomization 6 A brief History of Biotech