Emergence of Diverse Life Cycles and Life Histories at the Origin of Multicellularity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The History of Planet Earth
SECOND EDITION Earth’s Evolving The History of Systems Planet Earth Ronald Martin, Ph.D. University of Delaware Newark, Delaware © Jones & Bartlett Learning, LLC, an Ascend Learning Company. NOT FOR SALE OR DISTRIBUTION 9781284457162_FMxx_00i_xxii.indd 1 07/11/16 1:46 pm World Headquarters Jones & Bartlett Learning 5 Wall Street Burlington, MA 01803 978-443-5000 [email protected] www.jblearning.com Jones & Bartlett Learning books and products are available through most bookstores and online booksellers. To contact Jones & Bartlett Learning directly, call 800-832-0034, fax 978-443-8000, or visit our website, www.jblearning.com. Substantial discounts on bulk quantities of Jones & Bartlett Learning publications are available to corporations, professional associations, and other qualified organizations. For details and specific discount information, contact the special sales department at Jones & Bartlett Learning via the above contact information or send an email to [email protected]. Copyright © 2018 by Jones & Bartlett Learning, LLC, an Ascend Learning Company All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission from the copyright owner. The content, statements, views, and opinions herein are the sole expression of the respective authors and not that of Jones & Bartlett Learning, LLC. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not constitute or imply its endorsement or recommendation by Jones & Bartlett Learning, LLC, and such reference shall not be used for advertis- ing or product endorsement purposes. -

Nature Based Solutions FEMA
PROMOTING NATURE-BASED HAZARD MITIGATION THROUGH FEMA MITIGATION GRANTS ABBREVIATIONS ADCIRC – Advanced Circulation Model HGM Approach – Hydrogeomorphic Approach BCA – Benefit-Cost Analysis HMA – Hazard Mitigation Assistance BCR – Benefit-Cost Ratio HMGP – Hazard Mitigation Grant Program BRIC – Building Resilient Infrastructure and MSCP – Multiple Species Conservation Program Communities NBS – Nature-Based Solution C&CB – Capability- and Capacity-Building NFIP – National Flood Insurance Program CDBG-DR – Community Development Block Grant- Disaster Recovery NFWF – National Fish and Wildlife Foundation CDBG-MIT – Community Development Block NOAA – National Oceanic and Atmospheric Grant-Mitigation Administration D.C. – District of Columbia NOFO – Notice of Funding Opportunity DEM – Department of Emergency Management NPV – Net Present Value DOI – Department of the Interior SCC – State Coastal Conservancy EDYS – Ecological Dynamics Simulation SDG&E – San Diego Gas & Electric EMA – Emergency Management Agency SFHA – Special Flood Hazard Area EPA SWMM – Environmental Protection Agency SHMO – State Hazard Mitigation Officer Storm Water Management Model SLAMM – Sea Level Affecting Marshes Model FEMA – Federal Emergency Management Agency SRH-2D – Sedimentation and River Hydraulics – FIRM – Flood Insurance Rate Map Two-Dimension FMA – Flood Mitigation Assistance STWAVE – Steady-State Spectral Wave Model FMAG – Fire Management Assistance Grant TNC – The Nature Conservancy HAZUS – Hazards US USACE – U.S. Army Corps of Engineers HEC-HMS – Hydrologic -

The Solar System
5 The Solar System R. Lynne Jones, Steven R. Chesley, Paul A. Abell, Michael E. Brown, Josef Durech,ˇ Yanga R. Fern´andez,Alan W. Harris, Matt J. Holman, Zeljkoˇ Ivezi´c,R. Jedicke, Mikko Kaasalainen, Nathan A. Kaib, Zoran Kneˇzevi´c,Andrea Milani, Alex Parker, Stephen T. Ridgway, David E. Trilling, Bojan Vrˇsnak LSST will provide huge advances in our knowledge of millions of astronomical objects “close to home’”– the small bodies in our Solar System. Previous studies of these small bodies have led to dramatic changes in our understanding of the process of planet formation and evolution, and the relationship between our Solar System and other systems. Beyond providing asteroid targets for space missions or igniting popular interest in observing a new comet or learning about a new distant icy dwarf planet, these small bodies also serve as large populations of “test particles,” recording the dynamical history of the giant planets, revealing the nature of the Solar System impactor population over time, and illustrating the size distributions of planetesimals, which were the building blocks of planets. In this chapter, a brief introduction to the different populations of small bodies in the Solar System (§ 5.1) is followed by a summary of the number of objects of each population that LSST is expected to find (§ 5.2). Some of the Solar System science that LSST will address is presented through the rest of the chapter, starting with the insights into planetary formation and evolution gained through the small body population orbital distributions (§ 5.3). The effects of collisional evolution in the Main Belt and Kuiper Belt are discussed in the next two sections, along with the implications for the determination of the size distribution in the Main Belt (§ 5.4) and possibilities for identifying wide binaries and understanding the environment in the early outer Solar System in § 5.5. -
Glossary of Terms
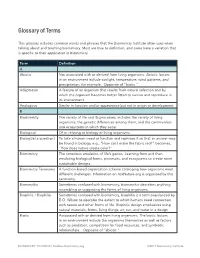
Glossary of Terms This glossary includes common words and phrases that the Biomimicry Institute often uses when talking about and teaching biomimicry. Most are true to definition, and some have a variation that is specific to their application in biomimicry. Term Definition A Abiotic Not associated with or derived from living organisms. Abiotic factors in an environment include sunlight, temperature, wind patterns, and precipitation, for example. Opposite of "biotic." Adaptation A feature of an organism that results from natural selection and by which the organism becomes better fitted to survive and reproduce in its environment. Analogous Similar in function and/or appearance but not in origin or development. B Biodiversity The variety of life and its processes; includes the variety of living organisms, the genetic differences among them, and the communities and ecosystems in which they occur. Biological Of or relating to biology or living organisms. Biologize [a question] To take a human need or function and rephrase it so that an answer may be found in biology, e.g., "How can I make the fabric red?" becomes, "How does nature create color?" Biomimicry The conscious emulation of life’s genius. Learning from and then emulating biological forms, processes, and ecosystems to create more sustainable designs. Biomimicry Taxonomy A function-based organization scheme cataloging how organisms meet different challenges. Information on AskNature.org is organized by this taxonomy. Biomorphic Sometimes confused with biomimicry, biomorphic describes anything resembling or suggesting the forms of living organisms. Biophilic / Biophilia Sometimes confused with biomimicry, biophilia is a term popularized by E.O. Wilson to describe the extent to which humans need connection with nature and other forms of life. -

Conceptions of the Nature of Biology Held by Senior Secondary School
Malaysian Online Journal of Educational Sciences 2017 (Volume5 - Issue 3 ) Conceptions of the Nature of Biology [1] Department of Science Education, University of Ilorin, Held by Senior Secondary School Ilorin, Nigeria P.M.B. 1515 Ilorin, Nigeria Biology Teachers in Ilorin, Kwara State, [email protected] Nigeria [2] Department of Science Education, University of Ilorin, Ilorin, Nigeria P.M.B. 1515 Ilorin, Nigeria Adegboye, Motunrayo Catherine[1], Ganiyu Bello [2], Isaac, O. [email protected] Abimbola[3] [3] Department of Science Education, University of Ilorin, Ilorin, Nigeria P.M.B. 1515 Ilorin, Nigeria ABSTRACT [email protected] There is a sustained public outcry against the persistent abysmal performance of students in biology and other science subjects at the Senior School Certificate Examinations conducted by the West African Examinations Council (WAEC) and the National Examinations Council (NECO). Biology is a unique science discipline with peculiar philosophical principles and methodology that are not applicable to other science disciplines. Understanding the unique structure of knowledge, principles and methodology for providing explanations in biology is sine qua-non for effective and efficiency teaching of biology by teachers, and meaningful learning by the students. This study, therefore, investigated the conceptions of the nature of biology held by biology teachers in Ilorin, Nigeria. The study adopted the descriptive research design of the survey type. A questionnaire entitled “Biology Teachers’ Conceptions of the Nature of Biology Questionnaire”(BTCNBQ) was designed by the researchers and used as the instrument for data collection. The population for the study comprised all the biology teachers in Ilorin, Nigeria. Simple random sampling technique was used to select two hundred and sixty (260) biology teachers from Ilorin, Nigeria. -

Origin and Evolution of Earth Research Questions for a Changing Planet
Origin and Evolution of Earth Research Questions for a Changing Planet Questions about the origins and nature of Earth have long preoccupied human thought and the scientific endeavor. Deciphering the planet’s history and processes could improve the abil- ity to predict catastrophes like earthquakes and volcanoes, to manage Earth’s resources, and to anticipate changes in climate and geologic processes. This report captures, in a series of questions, the essential scientific challenges that constitute the frontier of Earth science at the start of the 21st century. arth is an active place. Earthquakes rip along plate boundaries, volcanoes spew fountains of Emolten lava, and mountain ranges and seabed are constantly created and destroyed. Earth scientists have long been concerned with deciphering the history—and predicting the future—of this active planet. Over the past four decades, Earth scientists have made great strides in understanding Earth’s workings. Scientists have ever-improving tools to understand how Earth’s internal processes shape the planet’s surface, how life can be sustained over billions of years, and how geological, biological, atmospheric, and oceanic NASA/NDGC processes interact to produce climate—and climatic change. At the request of the U.S. Department of Energy, Na- tional Aeronautics and Space Administration, National Science Foundation, and U.S. Geological Survey, the National Research Council assembled a committee to propose and explore grand ques- tions in Earth science. This report, which is the result of the committee’s deliberations and input solicited from the Earth science community, describes ten “big picture” Earth science issues being pursued today. -

Human-Induced Alteration Negatively Impacts Perceived Naturalness and Preferences for Natural Environments Ethan A
Western Oregon University Digital Commons@WOU Faculty Research Publications (All Departments) Faculty Research 3-2016 Nature with a Human Touch: Human-Induced Alteration Negatively Impacts Perceived Naturalness and Preferences for Natural Environments Ethan A. McMahan Western Oregon University, [email protected] Jaime M. Cloud Western Oregon University Patrick Josh Western Oregon University Michaell Scott Western Oregon University Follow this and additional works at: https://digitalcommons.wou.edu/fac_pubs Part of the Psychology Commons Recommended Citation McMahan, E. A., Cloud, J. M., Josh, P., & Scott, M. (2016). Nature with a Human Touch: Human-Induced Alteration Negatively Impacts Perceived Naturalness and Preferences for Natural Environments. Ecopsychology, 8 (1). http://dx.doi.org/10.1089/ eco.2015.0068 This Article is brought to you for free and open access by the Faculty Research at Digital Commons@WOU. It has been accepted for inclusion in Faculty Research Publications (All Departments) by an authorized administrator of Digital Commons@WOU. For more information, please contact [email protected]. Running head: HUMAN ALTERATION, NATURALNESS, AND PREFERENCE 1 Nature with a Human Touch: Human-Induced Alteration Negatively Impacts Perceived Naturalness and Preferences for Natural Environments Ethan A. McMahana, Jaime M. Clouda, Patrick Josha, and Michaell Scotta a Department of Psychological Sciences, Western Oregon University, Monmouth, Oregon Correspondence regarding this article should be sent to Ethan McMahan, Department of Psychological Sciences, Western Oregon University, 345 N. Monmouth Ave., Monmouth, Oregon 97361. Email: [email protected]. HUMAN ALTERATION, NATURALNESS, AND PREFERENCE 2 Abstract Natural environments vary in the degree to which humans have altered them; some environments, like wilderness areas, are relatively untouched, while others, like urban green spaces, are heavily manicured. -

Biodiversity Glossary
BIODIVERSITY GLOSSARY Biodiversity Glossary1 Access and benefit-sharing One of the three objectives of the Convention on Biological Diversity, as set out in its Article 1, is the “fair and equitable sharing of the benefits arising out of the utilization of genetic resources, including by appro- priate access to genetic resources and by appropriate transfer of relevant technologies, taking into account all rights over those resources and to technologies, and by appropriate funding”. The CBD also has several articles (especially Article 15) regarding international aspects of access to genetic resources. Alien species A species occurring in an area outside of its historically known natural range as a result of intentional or accidental dispersal by human activities (also known as an exotic or introduced species). Biodiversity Biodiversity—short for biological diversity—means the diversity of life in all its forms—the diversity of species, of genetic variations within one species, and of ecosystems. The importance of biological diversity to human society is hard to overstate. An estimated 40 per cent of the global economy is based on biologi- cal products and processes. Poor people, especially those living in areas of low agricultural productivity, depend especially heavily on the genetic diversity of the environment. Biodiversity loss From the time when humans first occupied Earth and began to hunt animals, gather food and chop wood, they have had an impact on biodiversity. Over the last two centuries, human population growth, overex- ploitation of natural resources and environmental degradation have resulted in an ever accelerating decline in global biodiversity. Species are diminishing in numbers and becoming extinct, and ecosystems are suf- fering damage and disappearing. -

Waukesha County Parks and Land Use to Celebrate Earth Day All Month
Paul Farrow Dale Shaver County Executive Director Waukesha County Department of Parks and Land Use Contact: Emily Heller FOR IMMEDIATE RELEASE Phone: 262-548-7803 Date: Thursday, April 1, 2021 WAUKESHA COUNTY PARKS AND LAND USE TO CELEBRATE EARTH DAY THROUGHOUT APRIL (Waukesha, WI) – Waukesha County Parks and Land Use invites residents and visitors to celebrate Earth Day every day throughout the month of April with great activities and events that focus on our planet, sustainability, and conservation for all ages. “We feel that Earth Day should be every day!” said Janet Barthel, Retzer Nature Center Supervisor. “We love engaging with people to show them how their actions matter. Even small actions can make a positive impact.” Retzer Environmental Learning Center will host their first “Story Hike” in partnership with Bridges Library System. This new activity combines reading and hiking in nature for families and individuals. The April “Story Hike” is a self-guided hike on a trail featuring the book, 'Thank You, Earth – A Love Letter to Our Planet' by April Pulley Sayre. Retzer also encourages everyone to participate in the 30-Day Earth Challenge. Download the free calendar and see thirty simple actions to help our planet. More information is available at www.waukeshacounty.gov/earthweek. The Waukesha County Park System is also highlighting volunteer events taking place in the parks. Participants can help eradicate the invasive weed, garlic mustard, at Mission: Possible on April 24 or plant native trees at Menomonee Park for Arbor Day on April 30. Additional activities are taking place throughout the month. Event listings can be in the schedule below or online at www.waukeshacounty.gov/earthweek. -

EARTH SYSTEM: HISTORY and NATURAL VARIABILITY – Vol
EARTH SYSTEM: HISTORY AND NATURAL VARIABILITY – Vol. I - Earth System: History and Natural Variability - Vaclav Cilek, Rachel H. Smith EARTH SYSTEM: HISTORY AND NATURAL VARIABILITY Vaclav Cilek Institute of Geology ASCR, Praha, Czech Republic Rachel H. Smith Department of Linguistics, Cambridge, UK Keywords: Earth system, survival, biodiversity, geological history, paleoclimate, global cycles, origin of life, future of Earth system Contents 1. Introduction: Earth as one of the Planets in the Solar System 2. Origin and History: The Earth Does not Stand Still 3. The Earth’s Internal Structure: Deep Causes of Surficial Processes 4. The Emergence of Life: the Life of Emergence 5. Biodiversity: A Geological Perspective 6. Climate: Dialogue between the Planet and Life 7. Global cycles: Unity in Diversity of Phenomena 8. Future: The Wisdom to Observe, the Will to Change Acknowledgements Glossary Bibliography Biographical Sketches Summary “The Earth is all the home I have”. W.E. Aytoun The Earth is one of the planets in the solar system, the third closest to the Sun and the fifth largest in diameter. It is the only planet known to support higher forms of life and civilization capable of self-reflection. The Earth is the home of innumerable plants, creatures and human beings, which are connected by an intricate web of mutual relations and feedback. The Earth is an almost spherical body with an equatorial radius of about 6378 km. Its outstanding feature is the presence of liquid water. Water is not only crucial for life but its cycle keeps the surface in a state of constant change due to erosion and sedimentation. -

Biodiversity Loss and Its Impact on Humanity. Nature
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/225283251 Biodiversity loss and its impact on humanity. Nature ARTICLE in NATURE · JUNE 2012 Impact Factor: 41.46 · DOI: 10.1038/nature11148 · Source: PubMed CITATIONS READS 763 1,479 17 AUTHORS, INCLUDING: Andrew Gonzalez Anita Narwani McGill University University of Michigan 118 PUBLICATIONS 6,074 CITATIONS 22 PUBLICATIONS 937 CITATIONS SEE PROFILE SEE PROFILE Michel Loreau French National Centre for Scientific Resea… 278 PUBLICATIONS 22,483 CITATIONS SEE PROFILE Available from: Michel Loreau Retrieved on: 10 February 2016 REVIEW doi:10.1038/nature11148 Biodiversity loss and its impacton humanity Bradley J. Cardinale1, J. Emmett Duffy2, Andrew Gonzalez3, David U. Hooper4, Charles Perrings5, Patrick Venail1, Anita Narwani1, Georgina M. Mace6, David Tilman7, David A. Wardle8, Ann P. Kinzig5, Gretchen C. Daily9, Michel Loreau10, James B. Grace11, Anne Larigauderie12, Diane S. Srivastava13 & Shahid Naeem14 The most unique feature of Earth is the existence of life, and the most extraordinary feature of life is its diversity. Approximately 9 million types of plants, animals, protists and fungi inhabit the Earth. So, too, do 7 billion people. Two decades ago, at the first Earth Summit, the vast majority of the world’s nations declared that human actions were dismantling the Earth’s ecosystems, eliminating genes, species and biological traits at an alarming rate. This observation led to the question of how such loss of biological diversity will alter the functioning of ecosystems and their ability to provide society with the goods and services needed to prosper. n the past 20 years remarkable progress has been made towards its role in ecosystem and landscape processes5. -

A Glossary for Avian Conservation Biology
This file was created by scanning the printed publication. Errors identified by the software have been corrected; however, some errors may remain. Wilson Bull., 106(1), 1994, pp. 121-137 A GLOSSARY FOR AVIAN CONSERVATION BIOLOGY ROLF R. KOFORD,' JOHN B. DUNNING, JR.,2 CHRISTINE A. RIBIC,3 AND DEBORAH M. FINCH4 ABSTRACT.-This glossary provides standard definitions for many of the terms used in avian conservation biology. We compiled these definitions to assist communication among researchers, managers, and others involved in the Neotropical Migratory Bird Conservation Program, also known as Partners in Flight. We used existing glossaries and recent literature to prepare this glossary. The cited sources were not necessarily the first ones to use the terms. Many definitions were taken verbatim from the cited source material. Others were modified slightly to clarify the meaning. Definitions that were modified to a greater extent are indicated as being adapted from the originals. Terms that have been used in more than one way by different authors are listed with numbered alternative definitions if the definitions differ substantially. Received 30 March 1993, accepted 23 July 1993. GLOSSARY Accuracy: the closeness of computations or estimates to the exact or true value (Marriott 1990:2). After-hatching-year (AHY) bird: a bird in at least its second calendar year of life (Pyle et al. 1987:27; Canadian Wildlife Service and U.S. Fish and Wildlife Service 1991:5-47). After-second-year (ASY) bird: a bird in at least its third calendar year of life (Pyle et al. 1987:27; Canadian Wildlife Service and U.S.