Weaving Charlotte's Web: an In-Depth Guide to Cannabidiol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coshocton County Agriculture & Natural Resources
OHIO STATE UNIVERSITY EXTENSION COSHOCTON COUNTY AGRICULTURE & NATURAL RESOURCES Hello Coshocton County! The 49th Annual Fall Foliage & Farm Tour was a huge success as over 1,800 people attended this year’s event in eastern Coshocton County. We appreciate all of our stops for all their work to make this event such a success. It was a wonderful tour! See the enclosed article for more information about the tour and to see some pictures from the event. We have been overwhelmed to the response we have received to the pre-sale of tickets for the 4th Annual “For the Love of Lamb Dinner” on Saturday, November 2 at 6:00 pm. In fact, we are October 23, 2019 Issue already SOLD-OUT as all 65 tickets for the event have been purchased! What a great response to this fabulous dinner. Fall Foliage & Farm Tour a Success Typical Autumn Weather Changes Harvest continues to roll along across the county. I was pleased Ahead that we were able to harvest the “Boots on the Ground” soybean Hay Quality Indicators research plot in conjunction with Lapp Farms on Sunday, October Consider By-product Feeds in Rations 13. We were pleased with the results which will be published in This Winter OSU eFields publication this fall. We will also be sharing these Ohio CAUV Values Projected to results at our winter Agronomy School this winter. Decline Through 2020 Ohio Proposed Hemp Rules are Set Considering Being a Master Gardener I have also been busy planning Extension events and workshops November Farmers Breakfast for this winter. -

Sunn Hemp Shines in New England
Sunn Hemp Shines in Massachusetts Sam Corcoran & Masoud Hashemi Foreground: Testing out sunn hemp as mulch and fertilizer for garlic, fall 2017; Background: a field of flowering sunn hemp before winterkill, early November. Sunn hemp is a new, summer crop for us in the Northeast. Despite its name, Sunn hemp (Crotalaria juncea) is not related to the industrial hemp you may be familiar with (Cannabis sativa). The Sunn Hemp plant bears only a mild resemblance to Cannabis, and is actually a legume in the same family as peas and beans. As a legume, Sunn Hemp has a relationship with bacteria that convert atmospheric nitrogen into plant-available nitrogen. It is believed that this tropical crop has been grown for hundreds of years, and it remains popular in India, Bangladesh, and Brazil. Sunn Hemp can be used for for- age, fiber, or as a green manure to provide nitrogen to subsequently planted crops. Modern interest in Sunn Hemp in the U.S. surged in Hawaii in the 80’s. Research spread across the southern U.S. in the 90’s through present, with Mid-Atlantic States also taking a recent research inter- est. Within just the past 2-3 years, a few seed companies have start- ed readily supplying Sunn Hemp throughout the U.S. Four years ago, we tried planting this crop at the UMass Research Farm and discovered we can grow Sunn Hemp, too. Despite our cooler climate, the hot summers in Massachusetts are sufficient for this tropical crop; in the 2016 drought, Sunn Hemp remained high performing while other crops suffered. -

Redondo CBD Report Fuller
CBD Recommended Local Public Health And Safety Regulations Prepared by Jonatan Cvetko Co-Founder Angeles Emeralds 1/21/2020 Table of Contents Introduction 3 CBD: Hemp vs Cannabis 4 Recommendation #1: Ban all Non-Cannabis Sourced CBD 5 1. Public Health Threat 5 News Reports of CBD Dangers 6 2. Prohibited by Federal Food and Drug Administration (FDA) 8 3. Prohibited by California Department of Public Health 8 4. Prohibited by Los Angeles County Department of Public Health 8 5. Coalition of Cities Successfully Opposed AB 228 9 6. Unregulated and Unsafe CBD is infiltrating Cities 10 7. No minimum Age Restrictions 13 8. Limit Liability 13 9. Reduces City Tax Revenues 13 10. CBD shops can be fronts of Illegal Cannabis Activities 14 Recommendation #2: Maintain Ban on Outside Cannabis Delivery Services and BAN POS/Kiosk transactions 16 Outside Delivery Uses CBD Shops as a loophole 16 POS/Kiosks provide loopholes for transactions 18 Examples of other jurisdictions regulations 20 Thousand Oaks 20 Moorpark 20 Hanford 20 Farmersville 20 Lynwood 20 Humboldt 20 Fortuna 21 Blue Lake 21 Summary 21 Page 2 of 21 INTRODUCTION Angeles Emeralds is an association of patients, advocates, business owners and other thoughtful stakeholders advocating for responsible cannabis policy with a particular focus on: 1) Protecting our children 2) Developing a pathway to licensure for existing responsible operators 3) Environmentally responsible sun grown cultivation 4) Social and Health equity Jonatan Cvetko is the Co-Founder of Angeles Emeralds is a member of the LA County Office of Cannabis Management Cannabis Advisory Working Group and serves in other advisory roles with other jurisdictions. -

Healing Herb Fitness High Stress Less
CENTENNIAL SPOTLIGHT CENTENNIAL SPOTLIGHT ® ® WOMEN WEED™ STRESS& LESS HEALING HERB Discover the Marijuana's Calm of CBD Medical Miracles COVID-19 FITNESS HIGH Why the Plant How THC WOMEN & WEED & WOMEN Can Help Boosts Workouts ™ PLUS Is Cannabis CENTENNIAL SPECIALS the Female Viagra? Display Until 4/26/21 $12.99 CENTENNIAL SPOTLIGHT CENTENNIAL SPOTLIGHT ® ® WOMEN WEED™ STRESS& LESS HEALING HERB Discover the Marijuana's Calm of CBD Medical Miracles COVID-19 FITNESS HIGH Why the Plant How THC WOMEN & WEED & WOMEN Can Help Boosts Workouts ™ PLUS Is Cannabis CENTENNIAL SPECIALS the Female Viagra? Display Until 4/26/21 $15.99 CENTENNIAL SPOTLIGHT® WOMEN &WEED™ 2 WOMEN & WEED 3 SECTION 1 34 CANNABIS PRIMER 8 News of the Weed World 14 Words of Weed 54 EDITOR’S LETTER 16 Terpenes & Cannabinoids 20 Seven Studies to Know Now So 2020 was … well, it was 24 State of Disunion something. Between the 28 What’s Legal Where COVID-19 pandemic, murder You Live hornets, civil unrest and an election like no other, it’s no wonder so many of us are SECTION 2 excited to dive headfirst into 2021. And things are looking HEALTH AND WELLNESS good…at least on the cannabis 34 The Wonder Weed front. In this issue, we’ll talk 40 CBD and Stress about how weed won big in the 44 Could CBD Be the November elections, with five Female Viagra? states passing measures to 46 Weed With Your Workout legalize medical or adult-use marijuana and more soon to 50 When Pot Isn’t 28 follow, plus what the Biden Working for You administration means for federal legalization. -

Impact of Hemp Legalization on Safety Oversight of Cmv Drivers Office of Drug and Alcohol Policy and Compliance (Odapc)
IMPACT OF HEMP LEGALIZATION ON SAFETY OVERSIGHT OF CMV DRIVERS OFFICE OF DRUG AND ALCOHOL POLICY AND COMPLIANCE (ODAPC) • Author and guardian of 49 CFR Part 40, throughout the Department: • Federal Aviation Administration (FAA) • Federal Motor Carrier Safety Administration (FMCSA) • Federal Railroad Administration (FRA) • Federal Transit Administration (FTA) • Pipeline and Hazardous Materials Safety Administration (PHMSA), as well as for the • US Coast Guard (USCG) • ODAPC provides intermodal coordination and ensures a ONE-DOT approach to regulatory, policy, and compliance matters. 2 DOT DRUG TESTING • Drug tests detect recent drug use - NOT impairment. • No impairment standard for drug levels like alcohol levels. • Windows of detection for each drug varies, but marijuana may be detected for up to 30 days based on the employee’s use and physical build. • DOT tests for: • Marijuana (THC) • Opioids (OPI) • Cocaine (COC) • Codeine • Amphetamines (AMP) • Morphine • Amphetamine • 6-AM (heroin) • Methamphetamine • Hydrocodone • MDMA • Hydromorphone • MDA • Oxycodone • Oxymorphone • Phencyclidine (PCP) 3 HEMP AS DEFINED BY THE 2018 FARM BILL Agricultural Marketing Act of 1946 [amended to include] Subtitle G— Hemp Production SEC. 297A. [7 U.S.C. 1639o] DEFINITIONS. (1) HEMP.—The term ‘‘hemp’’ means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis. cannabinoids = THC (Δ9-THC, Δ8-THC), CBD, CBV, CBN, CBC, CBG, CBL…. 4 HEMP VS MARIJUANA Hemp Marijuana • Cannabis sativa L. species • Cannabis sativa L. -

Sproutman's Hemp Sprouting
Sproutman’s Hemp Sprouting Bag Invented circa 1979 by Steve Meyerowitz, Sproutman® 1. Sterilize your new sprout bag by turning it inside cause problems. 3. Soak ½ cup of out and bathing it in boiling water for only 5 minutes. seed (see chart) in a jar overnight—- 2. Purchase seeds that are specifically adapted for about 8 hours—no more. Use a jar sprouting. Seeds from food store bulk bins typically with 16-32 ounces of pure water. Leave hanging or set in a bowl after dripping stops 4. After the 8 hrs., pour the soaked seeds into the wet, pre-washed sprout bag. Pull the draw string closed. Rinse by dipping the bag into a bowl of water or soaking it in the sink. Soak for at least 1 minute. Then hang it on a hook or knob or lay it in the dish rack or dishwasher rack. 5. Rinse twice per day, about 12 hours apart. Think of feeding them (watering) when you have breakfast and dinner. Just dip and hang! It only takes a minute! You’ve now got the basic steps. Variety #Grow Days Amount Skill Level Spelt 2-3 4-8 oz Easy Hard Wheat 2-3 4-8 oz Easy Kamut 2-3 4-8 oz Easy Soft Wheat 2-3 4-8 oz Easy Green Pea 4-5 4-8 oz Easy Lentil 4-5 4-8 oz Easy Mung 4-5 4-8 oz Easy Hulled Sunflower 2 4-8 oz Easy Radish 5-6 2-3 oz Easy Adzuki 4-5 4-8 oz Medium Broccoli 6 2-3 oz Medium Fenugreek 6 2-3 oz Medium Alfalfa 6-7 2-3 oz Medium Clover 6-7 2-3 oz Medium Chick Pea 4-5 4-8 oz Hard Soybean 4-5 4-8 oz Hard Chia 12 2-3 oz Very Hard About the Chart The sprout bag is very versatile and grows most sprout seeds. -

Hemp Testing Guidelines Issued January 15, 2021
Laboratory Testing Guidelines U.S. Domestic Hemp Production Program Issued January 15, 2021 Purpose: 1. Standard testing procedures are specified for samples taken in accordance with the Sampling Procedures for the USDA Hemp Production Program to measure the total delta-9 tetrahydrocannabinol (THC) concentration levels of samples on a dry weight basis. 2. The results are intended to measure the total THC concentration of composite hemp samples collected from a “lot” of hemp crop acreage designated by a hemp producer and as reported to USDA as required under the USDA Hemp Production Program. The purpose of the measurements is to determine whether the total THC concentration of the tested material is within the acceptable hemp THC level. Scope: 1. Hemp grown under a USDA, State, or Tribal hemp production plan is subject to sampling and compliance testing for THC concentration. Certain producers, including research institutions and facilities growing immature plants may have different testing requirements depending on the applicable State or Tribal plan and regulations. 2. Tests shall measure the total THC concentration in a sample submitted to a laboratory for analysis. The laboratory will perform chemical analysis on the sample using post- decarboxylation or other similarly reliable methods where the total THC concentration level considers the potential to convert delta-9-tetrahydrocannabinolic acid (THCA) into THC. 3. The total delta-9 tetrahydrocannabinol concentration level shall be determined and reported on a dry weight basis. 4. Laboratories shall calculate and include the Measurement of Uncertainty (MU) when they report THC concentration test results. “Measurement of uncertainty” is defined as “the parameter, associated with the result of a measurement, that characterizes the dispersion of the values that could reasonably be attributed to the particular quantity subject to measurement.” USDA does not establish or standardize an upper or lower boundary for general use by laboratories to calculate a measurement of uncertainty. -

A Multifaceted Approach to Address Variation in Cannabis Sativa
University of Northern Colorado Scholarship & Creative Works @ Digital UNC Dissertations Student Research 5-2019 A Multifaceted Approach to Address Variation in Cannabis Sativa Anna Louise Schwabe Follow this and additional works at: https://digscholarship.unco.edu/dissertations Recommended Citation Schwabe, Anna Louise, "A Multifaceted Approach to Address Variation in Cannabis Sativa" (2019). Dissertations. 554. https://digscholarship.unco.edu/dissertations/554 This Text is brought to you for free and open access by the Student Research at Scholarship & Creative Works @ Digital UNC. It has been accepted for inclusion in Dissertations by an authorized administrator of Scholarship & Creative Works @ Digital UNC. For more information, please contact [email protected]. © 2019 ANNA LOUISE SCHWABE ALL RIGHTS RESERVED UNIVERSITY OF NORTHERN COLORADO Greeley, Colorado The Graduate School A MULTIFACETED APPROACH TO ADDRESS VARIATION IN CANNABIS SATIVA A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Anna Louise Schwabe College of Natural and Health Sciences School of Biological Sciences Biological Education May 2019 This Dissertation by: Anna Louise Schwabe Entitled: A Multifaceted Approach to Address Variation in Cannabis sativa has been approved as meeting the requirement for the Degree of Doctor of Philosophy in College of Natural and Health Sciences in School of Biological Sciences, Program of Biological Education. Accepted by the Doctoral Committee ____________________________________________________ -
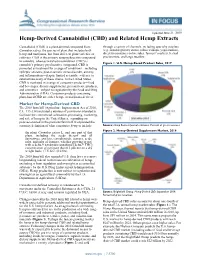
Hemp-Derived Cannabidiol (CBD) and Related Hemp Extracts
Updated June 21, 2019 Hemp-Derived Cannabidiol (CBD) and Related Hemp Extracts Cannabidiol (CBD) is a plant-derived compound from through a variety of channels, including specialty retailers Cannabis sativa, the species of plant that includes both (e.g., natural grocery stores, tobacco shops, yoga studios), hemp and marijuana, but from different plant varieties or direct-to-consumer online sales, farmers’ markets, herbal cultivars. CBD is the primary nonpsychoactive compound practitioners, and large retailers. in cannabis, whereas tetrahydrocannabinol (THC) is cannabis’s primary psychoactive compound. CBD is Figure 1. U.S. Hemp-Based Product Sales, 2017 promoted as treatment for a range of conditions—including epileptic seizures, post-traumatic stress disorder, anxiety, and inflammation—despite limited scientific evidence to substantiate many of these claims. In the United States, CBD is marketed in a range of consumer products—food and beverages, dietary supplements, personal care products, and cosmetics—subject to regulation by the Food and Drug Administration (FDA). Consumer products containing plant-based CBD are either hemp- or marijuana-derived. Market for Hemp-Derived CBD The 2018 farm bill (Agriculture Improvement Act of 2018, P.L. 115-334) included a number of provisions intended to facilitate the commercial cultivation, processing, marketing, and sale of hemp in the United States, expanding on policies enacted in the previous farm bill. It expanded the statutory definition of what constitutes hemp to include: Source: Hemp Business Journal estimates. Percent of gross revenues. Figure 2. Hemp-Derived Supplement Market, 2018 the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol [delta-9 THC] concentration of not more than 0.3 percent on a dry weight basis. -

TY18:Layout 1 17/08/09 16:06 Pagina 1 TY18:Layout 1 17/08/09 16:06 Pagina 2 TY18:Layout 1 17/08/09 16:06 Pagina 3 TY18:Layout 1 17/08/09 16:06 Pagina 4
TY18:Layout 1 17/08/09 16:06 Pagina 1 TY18:Layout 1 17/08/09 16:06 Pagina 2 TY18:Layout 1 17/08/09 16:06 Pagina 3 TY18:Layout 1 17/08/09 16:06 Pagina 4 Publisher/ Editor in Chief Marco Renda [email protected] Assistant to Editor Jef Tek [email protected] Copy Editor Aendrew Rininsland [email protected] Magazine design & layout Ivan Art [email protected] Director of Sales & Marketing Michelle Rainey [email protected] Technical Writer Ally a.k.a Pflover [email protected] Q&A Advisor Shantibaba [email protected] Text & photography Contributors Marco Renda, Ændrew Rininsland, Ivan Art, Michelle Rainey, Otto Snow, PFlover, Jef Tek, Shantibaba, Jerry B., Soma, Jay Generation, Harry Resin, Dr Dog, Chris Thompson, Jeremy Norrie, Keith Fagin, John (Shiva), Lara Lesack, Richard Owl Mirror, Suggarpaw, British Hempire, Jackie Sutton, Salvatore Messina HD., David B. Allen M.D., Hashmasta-Kut, Ale Keppel, Gregorio “Goyo” Fernandez Cover Pic `Mr Nice Critical Mass flower at 6 weeks` by Gregorio Fernandez “Goyo” for Mr. Nice Seedbank Submissions [email protected] Treating Yourself 250 The East Mall, P.O. Box 36531 Etobicoke, Ontario M9B 3Y8 Canada T: + 416 620 1951 F: +416 620 0698 Printed in Canada 4 - Treating Yourself, Issue 18- 2009 TY18:Layout 1 17/08/09 16:06 Pagina 5 Marco’s Editorial Well I have to say issue number 17 certainly caused a lot of controversy, especially surrounding the Dr Frankenbeanstien article. Both Sam The Skunkman and Ed Rosenthal contacted me regarding this. I offered them both the chance to rebut what was published, but they declined to do so. -

Hemp: a New Crop with New Uses for North America*
Reprinted from: Trends in new crops and new uses. 2002. J. Janick and A. Whipkey (eds.). ASHS Press, Alexandria, VA. Hemp: A New Crop with New Uses for North America* Ernest Small and David Marcus “Hemp” refers primarily to Cannabis sativa L. (Cannabaceae), although the term has been applied to dozens of species representing at least 22 genera, often prominent fiber crops. For examples, Manila hemp (abaca) is Musa textilis Née, sisal hemp is Agave sisalina Perrine, and sunn hemp is Crotolaria juncea L. Especially confusing is the phrase “Indian hemp,” which has been used both for narcotic Asian land races of C. sativa (so-called C. indica Lamarck of India) and Apocynum cannabinum L., which was used by North American Indians as a fiber plant. Cannabis sativa is a multi-purpose plant that has been domesticated for bast (phloem) fiber in the stem, a multi-purpose fixed oil in the “seeds” (achenes), and an intoxicating resin secreted by epidermal glands. The common names hemp and marijuana (much less frequently spelled mari- huana) have been applied loosely to all three forms, although historically hemp has been used primarily for the fiber cultigen and its fiber preparations, and marijuana for the drug cultigen and its drug preparations. The current hemp industry is making great efforts to point out that “hemp is not marijuana.” Italicized, Cannabis refers to the biological name of the plant (only one species of this genus is commonly recognized, C. sativa L.). Non-italicized, “cannabis” is a generic abstraction, widely used as a noun and adjective, and commonly (often loosely) used both for cannabis plants and/or any or all of the intoxicant preparations made from them. -

Autoimmune Protocol Food List
Autoimmune Protocol Food List The Paleo Autoimmune Protocol (AIP) eliminates certain primal foods that can sometimes trigger inflammation in people with autoimmune disease (dairy, eggs, nightshades, nuts and seeds). AIP isn’t necessarily easy – it definitely takes commitment. If you have an autoimmune condition and you want to get on top of it, then you’re going to need to do AIP 100%. We want everyone to be able to enjoy the benefits of our 10 Week Program and reclaim their health, so we have designed recipes to suit those following this protocol. We have also put together this supporting list of foods that are suitable for an Autoimmune Protocol. © PETE EVANS CHEF PTY LTD 2015. ALL RIGHTS RESERVED © PETE EVANS CHEF PTY LTD 2015. ALL RIGHTS RESERVED Vegetables & Fruits Protein Fats Organic is best Naturally pasture fed and sustainably raised EAT EAT EAT Vegetables of all kinds (8 – 14 cups / day) Quality meats (Pastured, grass-fed, organic) Avocados As much variety as much possible Poultry in moderation due to high omega-6 Coconut Colourful vegetables and fruit Beef Fatty fish Cruciferous vegetables Buffalo Pastured, grass-fed animal fats Arugula Chicken Olives Broccoli Duck Brussels sprouts Elk Probiotic foods Cabbage Lamb Kale Pheasant EAT Mustard greens Pork Coconut milk kefir Turnips Rabbit Coconut milk yogurt Watercress Turkey Fermented vegetables or fruit Sea Vegetables Venison Kombucha Excluding algae e.g. Chlorella, Spirulina Wild boar Water kefir Organ meat and offal NOTE: AVOID Aim for 5 times a week, the more the better You can also improve your intake of Nightshades Fish and shellfish (Wild is best) important trace minerals by switching to Himalayan pink salt or “dirty” sea salt.