Metal Inputs to Narragansett Bay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1940 1948 Thests Advisor
EXPEDITING VEHICULAR CIRCULATION IN THE PROVIDENCE, RHODE ISLAND AREA THROUGH THE USE OF TRANSIT by Donald M. Graham B. S. in C. E. Illinois Institute of Technology 1940 Submitted In Partial Fulfillment Of The Requirements For The Degree of MASTER OF CITY PLANNING at the Massachusetts Institute of Technology 1948 47\ - AA Signature of Author Department of City and Regional Planning September 17, .1948 Certified By Thests Advisor Providence, Rhode Island September 17, 1948 Professor F. J. Adams Department of City and Regional Planning School of Architecture Massachusetts Institute of Technology Cambridge, Massachusetts Dear Professor Adams. I hereby respectfully submit in partial fulfillment of the requirements for the degree of Master in City Planning my thesis, entitled "Expediting Vehicular Circulation in the Providence, Rhode Island Area Through the Use of Transit." Yours very truly, DONALD M. GRAHAM 2989is TABLE OF CONTEN'S Page. PREFACE... ... ... ... ..* .. ...00 ... 0 vi I. PREENT PHYSICAL PATTERN AND TRENDS 1. A. Physical Setting ... ... 1. B. Land Use .... .** .* 3. C. Population Density .. ... 4. D. Automotive Traffic .. 0.. ... 6. E. The Transit System ... ... ... 12. II. TRFATMENT OF TRENDS TO ENCOURAGE TRANSIT USE. 34. III. DOWNTOWN PROPOSALS TO ENCOURAGE TRANSIT USE. 45. A. Provision of a Through Transit System. U5. B. Segregation of Transit and Automotive Traffic ... 49. IV. BIBLIOGRAPHY .. .. a0 ..0 ..6*a 53. LIST OF TABLES Table Page I Number of Vehicles Entering and Leaving the Central Business District on a Weekday Between 8:00 a.m. and 6:00 p.m. .. .. .. 8. II The United Electric Railways Transit System in Providence and Pawtucket. .. 13. III Average Daily AM and PM Transit Passenger Counts for Three-Day Period July 22nd to 25th, 198. -

PROVIDENCE 2020 May 2006
PROVIDENCE 2020 may 2006 PROVIDENCE 2020 i ZHA | Barbara Sokoloff Associates | VHB Mayor of Providence David N. Cicilline I hope you fi nd this document as exciting as I do. In the pages that follow you will read about and see images of what our city may become. It is a future built on the best aspects of our past – reconnecting the city to the water, re-linking neighborhoods to the downtown, preserving our great stock or architecture, ensuring that transit serves a critical role in the life of the city, and creating superior public spaces. The goal of Providence 2020 is to provide a vision for coordinating and integrating the array of development opportunities in Providence. And given the unprecedented interest in our city and the unprecedented opportunities that will open up in the years ahead, a document of this type is critically important at this time. Providence 2020 details the principles that will guide us in the years ahead. The document spells out the priorities we will seek to achieve as we build and revitalize our city. It helps defi ne where development should take place, what the shape and character of development should be and what goals it should serve. It offers guidance for ways in which development can complement and promote public use and enjoyment of the city from streetscapes, to squares, to parks, to the waterfront. This is not a detailed blueprint of what Providence will look like in the future. That will unfold over time as projects are proposed and considered. But for those who want to make such proposals, this document provides the necessary guide. -

Rhode Island's Shellfish Heritage
RHODE ISLAND’S SHELLFISH HERITAGE RHODE ISLAND’S SHELLFISH HERITAGE An Ecological History The shellfish in Narragansett Bay and Rhode Island’s salt ponds have pro- vided humans with sustenance for over 2,000 years. Over time, shellfi sh have gained cultural significance, with their harvest becoming a family tradition and their shells ofered as tokens of appreciation and represent- ed as works of art. This book delves into the history of Rhode Island’s iconic oysters, qua- hogs, and all the well-known and lesser-known species in between. It of ers the perspectives of those who catch, grow, and sell shellfi sh, as well as of those who produce wampum, sculpture, and books with shell- fi sh"—"particularly quahogs"—"as their medium or inspiration. Rhode Island’s Shellfish Heritage: An Ecological History, written by Sarah Schumann (herself a razor clam harvester), grew out of the 2014 R.I. Shell- fi sh Management Plan, which was the first such plan created for the state under the auspices of the R.I. Department of Environmental Management and the R.I. Coastal Resources Management Council. Special thanks go to members of the Shellfi sh Management Plan team who contributed to the development of this book: David Beutel of the Coastal Resources Manage- Wampum necklace by Allen Hazard ment Council, Dale Leavitt of Roger Williams University, and Jef Mercer PHOTO BY ACACIA JOHNSON of the Department of Environmental Management. Production of this book was sponsored by the Coastal Resources Center and Rhode Island Sea Grant at the University of Rhode Island Graduate School of Oceanography, and by the Coastal Institute at the University SCHUMANN of Rhode Island, with support from the Rhode Island Council for the Hu- manities, the Rhode Island Foundation, The Prospect Hill Foundation, BY SARAH SCHUMANN . -

Oliver Stedman Government Center
100. COASTAL RESOURCE MANAGEMENT GOALS FOR PROVIDENCE HARBOR 110. PROVIDENCE HARBOR: A SPECIAL AREA OF CONCERN TO RHODE ISLAND Providence Harbor is the state's largest urban waterfront, reaching from Sabin Point and the Pawtuxet River northward to the falls at the head of the Seekonk River (Figure 1). It is located in the heart of the Providence metropolitan area, at the confluence of the major rivers and streams which drain a 1500 square kilometer basin inhabited by nearly one million people. The Seekonk and Providence Rivers, which are completely tidal, deliver both freshwater and pollutants associated with human activity and natural processes in the drainage basin directly to Upper Narragansett Bay, which is part of one of the most important estuaries in the United States. Industrialization and urban development have caused significant changes to Providence Harbor as an ecosystem, and as a place for Rhode Islanders to live and work. Providence Harbor is presently in transition as a place of importance to our economy and quality of life. Many problems persist as a consequence of the gradual weakening of the strength and vitality of the Providence metropolitan area, while new opportunities are appearing as public ownership of shorefront land has increase and a massive effort to control water pollution begins. The Rhode Island Coastal Resources Management Council (CRMC) is the state's primary agency for planning and management in the coastal zone. The Coastal Resources Management Program Document, a set of findings and policies adopted in 1977, outlines the CRMC's role in finding solutions to port and urban waterfront problems. -

Environmental Roundtable Newsletter, February 2005
RHODE ISLAND DEPARTMENT OF ENVIRONMENTAL MANAGEMENT February Protecting, Managing, and Restoring the Quality of Rhode Island’s Environment 2005 Environmental Roundtable News Governor Proposes Budget Measures To Reduce Waste and Save Landfill Space Governor Carcieri’s 2005 Budget includes major strategies to increase recycling and extend the life of the Central Landfill: a $1 per ton surcharge for commercial trash disposal and a $9.34 per ton increase for municipal trash disposal in communities that recycle less than 20% of their trash. The $1 per ton increase on commercial trash deposited at the Central Landfill would be used by DEM to enhance compliance inspections at waste management facilities and to implement a self-inspection and certification program to increase recycling in the commercial sector. Although it is not known precisely how much commercial recycling occurs in Rhode Island, there is significant potential for increasing the level of recycling in the business sector. The Department has drafted the new approach to commercial recycling to replace the existing commercial recycling and reporting system, which has proven costly and impractical to enforce. Starting January 1, 2006, municipalities that do not achieve a 20% recycling rate would see tipping fees rise from $32 per ton to $41.34 per ton. Presently 12 of the 39 cities and towns meet the 20% recycling rate. The proposed change to the municipal tip fee structure is designed to encourage waste diversion measures such as pay-as-you-throw, automated col- lection systems and enforcement. Both budget initiatives are consistent with proposed waste reduction policies in the draft Comprehensive Solid Waste Management Plan. -

Washington Park Main Street Plan Benjamin Bergenholtz
Roger Williams University DOCS@RWU Historic Preservation Community Partnerships Center 2012 Washington Park Main Street Plan Benjamin Bergenholtz Derek Dandurand Valerie Fram Tracy Jonsson Kimberly Lindner See next page for additional authors Follow this and additional works at: http://docs.rwu.edu/cpc_preservation Part of the Business Commons, Historic Preservation and Conservation Commons, Public Economics Commons, Urban, Community and Regional Planning Commons, and the Urban Studies and Planning Commons Recommended Citation Bergenholtz, Benjamin; Dandurand, Derek; Fram, Valerie; Jonsson, Tracy; Lindner, Kimberly; Reid, Carolyn; Sevigny, D.J.; Skerry, Alexandra; Guimond, Timothy; Kourafas, Brooke; Murphy, Elise; Berry, Matt; Butler, Erik; Nerone, Kayla; Robinson, Arnold; Wells, Jeremy; Coon, Julie; and Cooper, Joel, "Washington Park Main Street Plan" (2012). Historic Preservation. Paper 3. http://docs.rwu.edu/cpc_preservation/3 This Document is brought to you for free and open access by the Community Partnerships Center at DOCS@RWU. It has been accepted for inclusion in Historic Preservation by an authorized administrator of DOCS@RWU. For more information, please contact [email protected]. Authors Benjamin Bergenholtz, Derek Dandurand, Valerie Fram, Tracy Jonsson, Kimberly Lindner, Carolyn Reid, D.J. Sevigny, Alexandra Skerry, Timothy Guimond, Brooke Kourafas, Elise Murphy, Matt Berry, Erik Butler, Kayla Nerone, Arnold Robinson, Jeremy Wells, Julie Coon, and Joel Cooper This document is available at DOCS@RWU: http://docs.rwu.edu/cpc_preservation/3 ROGER WILLIAMS UNIVERSITY School of Architecture, Art, and Historic Preservation A MAIN STREET IMPLEMENTATION PLAN FOR THE WASHINGTON PARK NEIGHBORHOOD IN PROVIDENCE, RHODE ISLAND Prepared by Roger Williams University HP 682L: Graduate Preservation Planning Workshop Spring 2012 ! This report was compiled as part of a semester long project by the HP682L Roger Williams University (RWU) Graduate Preservation Planning Workshop in the Spring of 2012, under the direction of Dr. -

Towards a Resilient Providence I S S U E S | I M P a C T S | I N I T I a T I V E S | I N F O R M a T I O N
TOWARDS A RESILIENT PROVIDENCE I S S U E S | I M P A C T S | I N I T I A T I V E S | I N F O R M A T I O N F E B R U A R Y 2 0 2 1 | P R O V I D E N C E R E S I L I E N C E P A R T N E R S H I P Towards a Resilient Providence: Issues, Impacts, Initiatives, Information A report by Pam Rubinoff, Megan Elwell, Sue Kennedy, and Noah Hallisey. Edited by Lesley Squillante. February 2021 Cover photograph © Nicole Capobianco Acknowledgements We thank the researchers, practitioners, and stakeholders who have contributed to this effort to strengthen resilience in Providence and throughout Rhode Island. Their time, expertise, and varied perspectives have been invaluable in shaping this report and leading the work of the Providence Resilience Partnership. Contributors Curt Spalding, Institute at Brown for Environment and Society Horsley Witten Group Funding and Guidance Providence Resilience Partnership Personal Communications, Reviews, Information Monica Allard Cox, Rhode Island Sea Grant Leah Bamburger, City of Providence Austin Becker, URI David Bowen, Narragansett Bay Commission Rachel Calabro, RIDOH Al Dahlberg, Brown University Clara Decerbo, City of Providence David DosReis, City of Providence Barnaby Evans, WaterFire David Everett, City of Providence Janet Freedman, CRMC Tom Giordano, Partnership for Rhode Island Lichen Grewer, Brown University Meg Kerr, Audubon Society of Rhode Island Jo Lee, Brown University, PopUp Rhody Alicia Lehrer, Woonasquatucket River Watershed Council Isaac Ginis, URI Dan Goulet, CRMC Meg Goulet, Narragansett Bay Commission Jon McPherson, USACE Bonnie Nickerson, City of Providence Shaun O’Rourke, RI Infrastructure Bank Bill Patenaude, RIDEM Michael Riccio, USACE Elizabeth Scott, Elizabeth Scott Consulting Carolyn Skuncik, I-195 Commission Elizabeth Stone, RIDEM Tom Uva, Narragansett Bay Commission Chris Waterson, ProvPort | ii Foreword A Comprehensive Look at Providence’s Vulnerability Dear Fellow Rhode Islanders: We have both good news and difficult realities to present. -

The Providence Waterfront Guideplan
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 1991 THE PROVIDENCE WATERFRONT GUIDEPLAN Bryant Mitchell Keith University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Keith, Bryant Mitchell, "THE PROVIDENCE WATERFRONT GUIDEPLAN" (1991). Open Access Master's Theses. Paper 560. https://digitalcommons.uri.edu/theses/560 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. 1HE PROVIDENCE WATERFRONT GUIDEPLAN BY BRYANT MITCHELL KEITH A RESEARCH PROJECT SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF COMMUNITY PLANNING UNIVERSITY OF RHODE ISLAND 1991 Dedjcatjon This work is dedicated to my wife, Miriam, in humble recognition of her character and selfless support which made this effort possible. Providence Waterfront Guideplan TABLE OF CONTENTS Table of Contents .................................... List of Figures . iii List of Maps . iv Chapter 1: INTRODUCTION . I 1.1 Topic of Research . 1 1.2 Purpose of Study . 2 1.3 Methodology . 3 1.4 Scope of Study . 4 1.5 Organization of Study . 4 1.6 Definitions . 5 Chapter 2: OYERYIEW OF PROVIDENCE WATERFRONT STUDIES, Page 9 2.1 Area #1 - The Port of Providence Area . 9 2.2 Area #2 - The Downtown Waterfront Area ................. 19 1. The Old Harbor Section 2. The India Point/Fox Point Section 3. The Seekonk River Section 2.3 Other Relevant Studies .............................. 30 2.4 Evaluation of Existing Studies . -

165 Ferc ¶ 61031 United States of America
165 FERC ¶ 61,031 UNITED STATES OF AMERICA FEDERAL ENERGY REGULATORY COMMISSION Before Commissioners: Kevin J. McIntyre, Chairman; Cheryl A. LaFleur, Neil Chatterjee, and Richard Glick. National Grid LNG LLC Docket No. CP16-121-000 ORDER ISSUING CERTIFICATE (Issued October 17, 2018) On April 1, 2016, National Grid LNG LLC (National Grid) filed an application under section 7(c) of the Natural Gas Act (NGA)1 and Part 157 of the Commission’s regulations2 requesting authorization to add liquefaction facilities at its existing Fields Point liquefied natural gas (LNG) storage facility in Providence, Rhode Island. Currently, natural gas to be stored at National Grid’s facility must be trucked in from other facilities as LNG. The proposed Fields Point Liquefaction Project would enable customers to also transport gas as vapor by pipeline to Fields Point for liquefaction and storage. For the reasons discussed below, the Commission will grant National Grid’s requested certificate authorization, subject to conditions. I. Background and Proposal National Grid is a limited liability company organized under the laws of Delaware. National Grid owns and operates a 600,000-barrel of LNG (approximately 2 billion cubic feet of natural gas) capacity LNG storage facility at Fields Point on the Providence River in Providence, Rhode Island, and provides LNG storage, vaporization, and redelivery services subject to the Commission’s jurisdiction. Customers currently truck LNG to the Fields Point facility for storage, and National Grid redelivers gas to those customers, most often as vapor via pipeline, and on occasion as LNG by truck. Narragansett Electric Company (Narragansett Electric)3 pipeline facilities are used to deliver revaporized gas 1 15 U.S.C. -

Providence Tomorrow: Waterfront Plan
The waterfront of a great city is truly a special place. Providence’s waterfront along Narragansett Bay is no exception. Yet, like many waterfronts across the country, it is an area in transition, with large vacant and underutilized areas and few new activities to draw people to the water. i ii Introduction On May 31, 2006, Mayor Cicilline and the City Council announced Providence Tomorrow – an innovative and inclusive planning process designed to provide a framework for the growth and preservation of Providence neighborhoods. Since then, the City Council has adopted a new Comprehensive Plan and the Department of Planning and Development has conducted detailed planning studies in each of the city’s neighborhoods. During the development of the Comprehensive Plan, it became clear that the waterfront is an area of special value, of particular concern and interest to the City. As a result, the City initiated a special planning study and public charrette dedicated to the waterfront. This plan is a result of the planning study, outlining the goals and policies for the waterfront, and detailing the recommended zoning and regulatory changes for the study area. The City’s objectives in undertaking a comprehensive study of its waterfront were to revitalize the area, to promote economic development and jobs, and to increase municipal revenue through expansion of the tax base. The planning study had several components. In the spring of 2008, Ninigret Partners, PARE Engineering and LOCAL Architecture were hired to evaluate the economic conditions that will impact the Providence waterfront over the next two decades and to analyze the constraints to future development in greater detail. -

FIELDS POINT LIQUEFACTION PROJECT Environmental Assessment
20180625-3009 FERC PDF (Unofficial) 06/25/2018 National Grid LNG, LL FIELDS POINT LIQUEFACTION PROJECT Federal Energy C Regulatory Docket No. CP16-121-000 Commission Environmental Assessment Office of Energy Projects June 2018 Cooperating Agencies: Washington, DC 20426 20180625-3009 FERC PDF (Unofficial) 06/25/2018 20180625-3009 FERC PDF (Unofficial) 06/25/2018 FEDERAL ENERGY REGULATORY COMMISSION WASHINGTON, D.C. 20426 OFFICE OF ENERGY PROJECTS In Reply Refer To: OEP/DG2E/Gas 4 National Grid LNG, LLC Fields Point Liquefaction Project Docket No. CP16-121-000 TO THE PARTY ADDRESSED: The staff of the Federal Energy Regulatory Commission (FERC or Commission) has prepared an environmental assessment (EA) for the Fields Point Liquefaction Project, proposed by National Grid LNG, LLC (National Grid) in the above-referenced docket. National Grid requests authorization to construct natural gas liquefaction facilities at its existing Fields Point liquefied natural gas (LNG) storage facility in Providence, Rhode Island. The EA assesses the potential environmental effects of the construction and operation of the Fields Point Liquefaction Project in accordance with the requirements of the National Environmental Policy Act (NEPA). The FERC staff concludes that approval of the proposed project, with appropriate mitigating measures, would not constitute a major federal action significantly affecting the quality of the human environment. The U.S. Department of Transportation, Rhode Island Department of Environmental Management, and the Rhode Island Coastal Resources Management Council participated as cooperating agencies in the preparation of the EA. Cooperating agencies have jurisdiction by law or special expertise with respect to resources potentially affected by the proposal and participate in the NEPA analysis. -
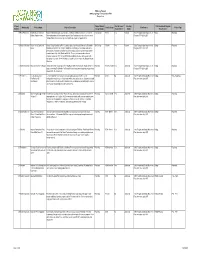
E. Pool of Projects
RI Moving Forward 2040 Long-range Transportation Plan Project List Project Project Status/ Cost at Year of Funding STIP Investment Program Municipality Project Name Project Description Cost Estimate Plan/Source Project Type Number Design Status Expenditure? Status Classification 15 East Providence Red Bridge (Henderson Major rehabilitation work, superstructure, and/or total bridge replacement. Includes Construction $91 M Yes Funded State Transportation Improvement Bridge Roadway Bridge) Replacement elminiating system of interchange ramps in East Providence to reduce the number of Program, FFY 2018-2027 transportation structures and open up land for development opportunities. 16 North Smithfield Route 146 at Sayles Hill Bridge Group 96 will bring RI-146 up to a state of good repair between I-295 and the 10% Design $150 M Yes Funded State Transportation Improvement Bridge Roadway Road Massachusetts State Line. Major rehabilitation and bridge preservation work will be Program, FFY 2018-2027 performed to extend the useful life of several bridges, and a new interchange will be constructed at Sayles Hill Road and RI-146. The project also includes a bus on shoulder system on RI-146 from I-95 to Mineral Spring Avenue, and several traffic safety improvements. RI-146 will also be resurfaced from I-295 to the Massachusetts State Line. 17 North Kingstown Route 403 Deferred Ramps Installation of two (2) ramps connecting RI-403 with West Davisville Road and one (1) Planning $100 M - $500 MYes Unfunded State Transportation Improvement Bridge Roadway ramp connecting RI-403 with Post Road. All three ramps were designed and permitted Program, FFY 2018-2027 as part of the RI-403 project.