Chestnut Blight" of Chinkapin in Florida1 E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHESTNUT (CASTANEA Spp.) CULTIVAR EVALUATION for COMMERCIAL CHESTNUT PRODUCTION
CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE By Ana Maria Metaxas Approved: James Hill Craddock Jennifer Boyd Professor of Biological Sciences Assistant Professor of Biological and Environmental Sciences (Director of Thesis) (Committee Member) Gregory Reighard Jeffery Elwell Professor of Horticulture Dean, College of Arts and Sciences (Committee Member) A. Jerald Ainsworth Dean of the Graduate School CHESTNUT (CASTANEA spp.) CULTIVAR EVALUATION FOR COMMERCIAL CHESTNUT PRODUCTION IN HAMILTON COUNTY, TENNESSEE by Ana Maria Metaxas A Thesis Submitted to the Faculty of the University of Tennessee at Chattanooga in Partial Fulfillment of the Requirements for the Degree of Master of Science in Environmental Science May 2013 ii ABSTRACT Chestnut cultivars were evaluated for their commercial applicability under the environmental conditions in Hamilton County, TN at 35°13ꞌ 45ꞌꞌ N 85° 00ꞌ 03.97ꞌꞌ W elevation 230 meters. In 2003 and 2004, 534 trees were planted, representing 64 different cultivars, varieties, and species. Twenty trees from each of 20 different cultivars were planted as five-tree plots in a randomized complete block design in four blocks of 100 trees each, amounting to 400 trees. The remaining 44 chestnut cultivars, varieties, and species served as a germplasm collection. These were planted in guard rows surrounding the four blocks in completely randomized, single-tree plots. In the analysis, we investigated our collection predominantly with the aim to: 1) discover the degree of acclimation of grower- recommended cultivars to southeastern Tennessee climatic conditions and 2) ascertain the cultivars’ ability to survive in the area with Cryphonectria parasitica and other chestnut diseases and pests present. -

The Effect of Insects on Seed Set of Ozark Chinquapin, Castanea Ozarkensis" (2017)
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 5-2017 The ffecE t of Insects on Seed Set of Ozark Chinquapin, Castanea ozarkensis Colton Zirkle University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/etd Part of the Botany Commons, Entomology Commons, and the Plant Biology Commons Recommended Citation Zirkle, Colton, "The Effect of Insects on Seed Set of Ozark Chinquapin, Castanea ozarkensis" (2017). Theses and Dissertations. 1996. http://scholarworks.uark.edu/etd/1996 This Thesis is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected], [email protected]. The Effect of Insects on Seed Set of Ozark Chinquapin, Castanea ozarkensis A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Entomology by Colton Zirkle Missouri State University Bachelor of Science in Biology, 2014 May 2017 University of Arkansas This thesis is approved for recommendation to the Graduate Council. ____________________________________ Dr. Ashley Dowling Thesis Director ____________________________________ ______________________________________ Dr. Frederick Paillet Dr. Neelendra Joshi Committee Member Committee Member Abstract Ozark chinquapin (Castanea ozarkensis), once found throughout the Interior Highlands of the United States, has been decimated across much of its range due to accidental introduction of chestnut blight, Cryphonectria parasitica. Efforts have been made to conserve and restore C. ozarkensis, but success requires thorough knowledge of the reproductive biology of the species. Other Castanea species are reported to have characteristics of both wind and insect pollination, but pollination strategies of Ozark chinquapin are unknown. -

Pooled Whole‐Genome Sequencing of Interspecific Chestnut (Castanea
Received: 3 April 2018 | Revised: 7 June 2018 | Accepted: 10 June 2018 DOI: 10.1002/ece3.4336 ORIGINAL RESEARCH Pooled whole- genome sequencing of interspecific chestnut (Castanea) hybrids reveals loci associated with differences in caching behavior of fox squirrels (Sciurus niger L.) Nicholas R. LaBonte1 | Keith E. Woeste2 1Department of Crop Sciences, University of Illinois, Urbana, Illinois Abstract 2USDA Forest Service, Northern Research Dispersal of seeds by scatter- hoarding rodents is common among tropical and tem- Station, Hardwood Tree Improvement perate tree species, including chestnuts in the genus Castanea. Backcrossed (BC) in- and Regeneration Center, West Lafayette, Indiana terspecific hybrid chestnuts exhibit wide variation in seed traits: as the parent species (Castanea dentata and C. mollissima) have distinct seed phenotypes and tend to be Correspondence Nicholas R. LaBonte, Department of Crop handled differently by seed dispersers, phenotypic variation in BC trees is likely due Sciences, University of Illinois, 1201 W to inheritance of genes that have undergone divergent evolution in the parent spe- Gregory Drive, Urbana, IL 61801. Email: [email protected] cies. To identify candidate genomic regions for interspecific differences in seed dis- persal, we used tagged seeds to measure average dispersal distance for seeds of third- generation BC chestnuts and sequenced pooled whole genomes of mother trees with contrasting seed dispersal: high caching rate/long distance; low caching rate/short distance; no caching. Candidate regions affecting seed dispersal were identified as loci with more C. mollissima alleles in the high caching rate/ long- distance pool than expected by chance and observed in the other two pools. Functional an- notations of candidate regions included predicted lipid metabolism, dormancy regu- lation, seed development, and carbohydrate metabolism genes. -

Phylogeny of Rosids! ! Rosids! !
Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Alnus - alders A. rubra A. rhombifolia A. incana ssp. tenuifolia Alnus - alders Nitrogen fixation - symbiotic with the nitrogen fixing bacteria Frankia Alnus rubra - red alder Alnus rhombifolia - white alder Alnus incana ssp. tenuifolia - thinleaf alder Corylus cornuta - beaked hazel Carpinus caroliniana - American hornbeam Ostrya virginiana - eastern hophornbeam Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Fagaceae (Beech or Oak family) ! Fagaceae - 9 genera/900 species.! Trees or shrubs, mostly northern hemisphere, temperate region ! Leaves simple, alternate; often lobed, entire or serrate, deciduous -
Native Plant List Trees.XLS
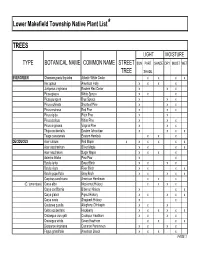
Lower Makefield Township Native Plant List* TREES LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE EVERGREEN Chamaecyparis thyoides Atlantic White Cedar x x x x IIex opaca American Holly x x x x Juniperus virginiana Eastern Red Cedar x x x Picea glauca White Spruce x x x Picea pungens Blue Spruce x x x Pinus echinata Shortleaf Pine x x x Pinus resinosa Red Pine x x x Pinus rigida Pitch Pine x x Pinus strobus White Pine x x x Pinus virginiana Virginia Pine x x x Thuja occidentalis Eastern Arborvitae x x x x Tsuga canadensis Eastern Hemlock xx x DECIDUOUS Acer rubrum Red Maple x x x x x x Acer saccharinum Silver Maple x x x x Acer saccharum Sugar Maple x x x x Asimina triloba Paw-Paw x x Betula lenta Sweet Birch x x x x Betula nigra River Birch x x x x Betula populifolia Gray Birch x x x x x Carpinus caroliniana American Hornbeam x x x (C. tomentosa) Carya alba Mockernut Hickory x x x x Carya cordiformis Bitternut Hickory x x x Carya glabra Pignut Hickory x x x x x Carya ovata Shagbark Hickory x x Castanea pumila Allegheny Chinkapin xx x Celtis occidentalis Hackberry x x x x x x Crataegus crus-galli Cockspur Hawthorn x x x x Crataegus viridis Green Hawthorn x x x x Diospyros virginiana Common Persimmon x x x x Fagus grandifolia American Beech x x x x PAGE 1 Exhibit 1 TREES (cont'd) LIGHT MOISTURE TYPE BOTANICAL NAME COMMON NAME STREET SUN PART SHADE DRY MOIST WET TREE SHADE DECIDUOUS (cont'd) Fraxinus americana White Ash x x x x Fraxinus pennsylvanica Green Ash x x x x x Gleditsia triacanthos v. -

Chinquapin Planting Guide
Planting Guide Food source: Chinkapin nuts are palatable to humans CHINKAPIN as well as wildlife. They have a sweet flavor and are often preferred over the fruit of the American Castanea pumila (L.) P. Mill. chestnut. Plant Symbol = CAPU9 Landscaping: Chinkapin is sometimes used for Contributed by: USDA NRCS National Plant Data landscaping as a small ornamental tree or shrub. Its Center flowers are attractive but have an unpleasant odor. Restoration: Chinkapin can be used to rehabilitate disturbed sites because of its ability to adapt to harsh conditions. The threat of chestnut blight often deters this decision by land managers. Wildlife: Squirrels, chipmunks, opossums, white- tailed deer, blue jays, woodpeckers and other birds consume chinkapin nuts. White-tailed deer browse Male flowers. A.B. Russell. 1997. the foliage. NC State University. Trees of the Maritime Forest. Legal Status Chinkapin is rare in its range. It is threatened in Kentucky, endangered in New Jersey, and has been extirpated from most of Alabama by chestnut blight. Please consult the PLANTS Web site and your State Department of Natural Resources for this plant’s current status (e.g. threatened or endangered species, state noxious status, and wetland indicator values). Description Female flowers. G. Nelson. 1996. General: Beech Family (Fagaceae). Chinkapin is a Shrubs and woody vines of Florida. monoecious small tree or large shrub that grows to be 2 to 5 m tall. The twigs are densely hairy Alternate Names (tomentose) when young, becoming shiny brown Allegheny chinkapin, American chinquapin, with densely reddish-hairy buds. The leaves are Castanea alnifolia, Castanea ashei, Castanea alternate, simple, short-stemmed, prominently floridana, Castanea margaretta, Castanea nana, veined, oblong with fine pointed teeth or bristles, up Castanea paucispina, chinquapin, dwarf chestnut, to 15 cm long, and tomentose on the lower surface. -

Calvert County Native Plant List
February 2011 CALVERT COUNTY NATIVE PLANT LIST Canopy Trees (Generally > 35 ft. tall at maturity) Planting Stock: 2-in. caliper in size spaced 20-40 ft. on center Common Name Species Notes Box Elder Acer negundo Red Maple Acer rubrum Silver Maple Acer saccharinum River Birch Betula nigra Bitternut Hickory Carya cordiformis Pignut Hickory Carya glabra Shagbark Hickory Carya ovata Mockernut Hickory Carya tomentosa Common Hackberry Celtis occidentalis Atlantic White Cedar Chamaecyparis thyoides Evergreen Common Persimmon Diospyros virginiana American Beech Fagus grandifolia Black Walnut Juglans nigra Eastern Red Cedar Juniperus virginiana Evergreen Sweet Gum Liquidambar styraciflua Tulip Poplar Liriodendron tulipifera Red Mulberry Morus rubra Black Gum Nyssa sylvatica Salt Tolerant Shortleaf Pine Pinus echinata Evergreen Pitch Pine Pinus rigida Evergreen; Salt Tolerant Pond Pine Pinus serotina Evergreen Loblolly Pine Pinus taeda Evergreen Virginia Pine Pinus virgiana Evergreen American Sycamore Platanus occidentalis Black Cherry Prunus serotina Salt Tolerant White Oak Quercus alba Salt Tolerant Swamp White Oak Quercus bicolor Salt Tolerant Scarlet Oak Quercus coccinea Salt Tolerant Southern Red Oak Quercus falcata Blackjack Oak Quercus marilandica Swamp Chestnut Oak Quercus michauxii Chinquapin Oak Quercus muehlenbergii Water Oak Quercus nigra Pin Oak Quercus palustris Salt Tolerant Willow Oak Quercus phellos Chestnut Oak Quercus prinus Northern Red Oak Quercus rubra Salt Tolerant Post Oak Quercus stellata Salt Tolerant Black Oak Quercus velutina Salt Tolerant Black Locust Robinia pseudoacacia Bald Cypress Taxodium distichum Salt Tolerant American Basswood Tilia americana American Elm Ulmus americana 1 February 2011 CAL VERT COUNTY NATIVE PLANTS LIST (cont.) Understory Trees (Generally < 35 ft. tall at maturity) Planting Stock: 1 to 2-in. -

Chinkapin Oak Quercus Muehlenbergii
Chinkapin Oak Quercus muehlenbergii Secondary Names: Chinquapin Oak Leaf Type: Deciduous Texas Native: Yes No Firewise: Yes No Tree Description: A medium or large tree reaching a height of 70 feet and a trunk to 3 feet in diameter, with a rounded crown of glossy, green foliage. It is also planted widely as a shade tree suitable for limestone soils. Range/Site Description: Occurs from northeast Texas to Central Texas and south to the Guadalupe River, and also in the mountains of West Texas, growing on mostly limestone soils, especially at the base of blus and along stream courses. Leaf: Simple, alternate, oval to elliptical or oblong in shape, 4" to 6" long and 1.5" to 2" wide, leaf edge rather sharply toothed but without bristle-tips, teeth slightly recurved. Flower: Separate male and female owers appear in spring on the same tree. Male owers borne on a yellowish catkin 3" to 4" long; the female owers are less conspicuous and reddish. Fruit: An acorn, requiring just one season to mature, 0.5" to 1.25" long, light to dark brown when ripe, enclosed by one-half its length byt the bowl-shaped cup. Acorn is edible if roasted. Bark: Light gray, breaking into short, narrow akes on the main trunk and limbs, deeply furrowed on older trunks. Wood: Heavy, hard, strong, durable, and taking an excellent polish; used for barrels, fencing, crossties, fuel, and occasionally for furniture. Similar Species: Swamp chestnut oak (Quercus michauxii) occurs in southeast Texas and has larger leaves with rounded teeth. Interesting Facts: Chinkapin oak is named because of the resemblance of the leaves to the Allegheny chinquapin (Castanea pumila), a relative of American chestnut (C. -
Native Plants of Maryland: What, When and Where
Home and Garden Mimeo HG#120 3/2005 Native Plants of Maryland: What, When and Where Eupatorium Cercis fistulosum canadensis Monarda didyma Rhododendron periclymenoides Tradescantia virginiana Tiarella cordifolia Rudbeckia hirta Lobelia cardinalis TABLE OF CONTENTS What are Native Plants ....................................... 2 Plant listings by preferred conditions .......... 15-20 Physiographic Map of Maryland ........................ 2 Plant Common Name Index ......................... 20-22 Invasive Non Natives .......................................... 3 References ........................................................ 23 Plant listing by type and preferences ............ 4-14 Glossary ............................................................ 23 Native Plants for Maryland INTRODUCTION WHAT ARE GROWTH CONDITIONS FOR NATIVE PLANTS? This guide is intended to help in the selection of native plants for habitat restoration, Maryland is host to a wide variety of native plants. This is due to the diversity of geo- critical area buffer management and natural landscaping projects. All of these plants graphical and climatic conditions. The state is divided into three physiographic regions are native to Maryland. Each section lists plants in alphabetical order by their Latin coastal, piedmont and mountain. You may use the map below to determine your region. names. Common names are included and are cross-referenced in the index. Growth conditions and plant characteristics are also included. State of Maryland Physiographic Regions WHAT ARE NATIVE PLANTS? A native plant is a species that originates or occurs naturally in a particular region. As our local habitat is disturbed by development, non-native and invasive plants change the character of our landscapes. Although many naturalized but introduced plants occur in most regions, the native plants listed are species that existed in Maryland when the European settlers arrived, or they are cultivars of these species. -

'Copper' Chinquapin
‘Copper’ chinquapin Castanea pumila (L.) P. Mill. A Conservation Plant Release by USDA NRCS Big Flats Plant Materials Center, Corning, New York Restoration: Copper can be used to rehabilitate disturbed sites because of its ability to adapt to harsh conditions. Food source: Copper nuts are palatable to humans as well as wildlife. They have a sweet flavor and are often preferred over the fruit of the American chestnut. Landscaping: Copper is sometimes used for landscaping as a small ornamental tree or shrub. It has long, dark green leaves and attractive flowers. Area of Adaptation and Use Copper chinquapin is adapted to the southern tier of New York to Florida. It can be grown on sandy loams to light ‘Copper’ chinquapin tree at the USDA NRCS Big Flats Plant clay soils. It is suitable to well-drained sites that receive Materials Center, in Big Flats, New York. full sun to partial shade. Typically found on dry uplands in deciduous or mixed woodlands. ‘Copper’ chinquapin was released in 2005 by USDA NRCS Big Flats Plant Materials Center. It was selected for its winter hardiness, superior plant vigor, and abundant fruit production. Description Copper is a native, deciduous, upland shrub belonging to the Beech family (Fagaceae), which grows to a mature height of 12 feet in its northern range and up to 20 feet, in the southern part. The oblong, alternate leaves are 6 inches long, simple, prominently veined with fine pointed teeth. Male flowers arise in the leaf axils, are yellow to white, and have a strong odor. Female flowers are rounder with a 1-inch diameter. -

Castanea Pumila Var. Ozarkensis (Ashe) Tucker, Comb
Journal of the Arkansas Academy of Science Volume 29 Article 23 1975 Castanea pumila var. ozarkensis (Ashe) Tucker, comb. Nov Gary E. Tucker Arkansas Tech University Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Botany Commons, and the Terrestrial and Aquatic Ecology Commons Recommended Citation Tucker, Gary E. (1975) "Castanea pumila var. ozarkensis (Ashe) Tucker, comb. Nov," Journal of the Arkansas Academy of Science: Vol. 29 , Article 23. Available at: http://scholarworks.uark.edu/jaas/vol29/iss1/23 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This Article is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Journal of the Arkansas Academy of Science, Vol. 29 [1975], Art. 23 Castaneapumila var ozarkensis (Ashe) Tucker, comb. nov. GARY E. TUCKER BiologyDepartment, Arkansas Polytechnic College, Russellville, Arkansas 72801 ABSTRACT Castanea ozarkensis Ashe, the Ozark chinquapin of the vascular plant family Fagaceae, is distributed widelythroughout the Interior Highlands of Arkansas and the adjacent states of Missouri and Oklahoma. Examination of material from throughout the range of C. ozarkensis indicates demonstrable morphological intergradation with C. -

Castanea Dentata
The American Chestnut: Breeding and Restoration KENDRA GURNEY TACF NEW ENGLAND REGIONAL BREEDING PROGRAM NOVEMBER 14, 2009 [email protected] American Chestnut 101 THE BASICS American chestnut: Castanea dentata y Member of the Fagaceae family { Beech (Fagus), chestnut (Castanea) and oak (Quercus) y Species of Castanea native to north America { Castanea dentata – American chestnut { Castanea pumila – Chinquapin or Allegheny Chinquapin { Castanea ozarkensis (Castanea pumila var. ozarkensis)– Ozark Chinquapin y Non-native Castanea species { Castanea mollissima – Chinese chestnut { Castanea crenata – Japanese chestnut { Castanea sativa – European chestnut { Castanea henryi – Henry’s chinquapin (China) { Castanea seguinii – Seguin chestnut (China) Historic Range { Most of MA { All of RI { Limited by cold 0r poor chestnut site conditions Simple, Alternate Deeply toothed, teeth hooked or curved in Narrow taper at base Small, pointed buds American Chestnut ID: Leaves Male and female flowers Self-infertile Flower in late June, after risk of frost Wind and insect pollinated American Chestnut ID: Flowers Densely spiny bur, more so than Horsechestnut Nuts three to a bur, large, brown and shiny Un-pollinated nuts are flat and rectangular American Chestnut ID: Burs and Nuts Timber-form Bark has wide, flat ridges American Chestnut ID: Mature Tree Root-collar sprouts are most common today American Chestnut ID: Root-Collar Sprouts Girdling, sunken stem cankers Orange fruiting bodies Water sprouts, drought symptoms American Chestnut ID: Blight (Cryphonectria