Phylogenomic Analysis of Picramnia, Alvaradoa, and Leitneria Supports the Independent Picramniales
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Redalyc.Tripanocide and Antibacterial Activity of Alvaradoa Subovata Cronquist Extracts
Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas ISSN: 0717-7917 [email protected] Universidad de Santiago de Chile Chile MARTÍNEZ, M. Laura; TRAVAINI, M. Lucía; RODRIGUEZ, M. Victoria; ORELLANO, Elena; NOCITO, Isabel; SERRA, Esteban; GATTUSO, Martha; CORTADI, Adriana Tripanocide and antibacterial activity of Alvaradoa subovata Cronquist extracts Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas, vol. 12, núm. 3, mayo, 2013, pp. 302-312 Universidad de Santiago de Chile Santiago, Chile Available in: http://www.redalyc.org/articulo.oa?id=85626383006 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative © 2013 Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 12 (3): 302 - 312 ISSN 0717 7917 www.blacpma.usach.cl Artículo Original | Original Article Tripanocide and antibacterial activity of Alvaradoa subovata Cronquist extracts [Actividad tripanocida y antibacteriana de extractos de Alvaradoa subovata Cronquist] M. Laura MARTÍNEZ1, M. Lucía TRAVAINI1, M. Victoria RODRIGUEZ1, Elena ORELLANO2, Isabel NOCITO3, Esteban SERRA3, Martha GATTUSO1 & Adriana CORTADI1. 1Área Biología Vegetal 2Área Biología Molecular 3Área Parasitología. Facultad de Ciencias Bioquímicas y Farmacéuticas Universidad Nacional de Rosario. Suipacha 531. S2002LRK Rosario, Argentina Contactos | Contacts: M. Laura MARTÍNEZ - E-mail address: [email protected] Abstract We studied antioxidant, antibacterial and tripanocide activities of Alvaradoa subovata extracts. The ethanolic extracts showed the greatest DPPH radical scavenging capacity, especially that of bark with an IC50 = 4.7 ± 0.18 µg/mL. -

Low Risk, Fruit Tree, Edible Fruit, Slow-Growing, Bird-Dispersed, Zoochorous
Family: Sapindaceae Taxon: Talisia esculenta Synonym: Sapindus esculenta A. St.-Hil. (basionym) Common Name: pitomba Questionaire : current 20090513 Assessor: Chuck Chimera Designation: L Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score -1 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- High substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- High high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 y 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 n 301 Naturalized beyond native range y = 1*multiplier (see n Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see n Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see n Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see n Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 405 Toxic to animals y=1, n=0 406 Host for recognized pests -

Well-Known Plants in Each Angiosperm Order
Well-known plants in each angiosperm order This list is generally from least evolved (most ancient) to most evolved (most modern). (I’m not sure if this applies for Eudicots; I’m listing them in the same order as APG II.) The first few plants are mostly primitive pond and aquarium plants. Next is Illicium (anise tree) from Austrobaileyales, then the magnoliids (Canellales thru Piperales), then monocots (Acorales through Zingiberales), and finally eudicots (Buxales through Dipsacales). The plants before the eudicots in this list are considered basal angiosperms. This list focuses only on angiosperms and does not look at earlier plants such as mosses, ferns, and conifers. Basal angiosperms – mostly aquatic plants Unplaced in order, placed in Amborellaceae family • Amborella trichopoda – one of the most ancient flowering plants Unplaced in order, placed in Nymphaeaceae family • Water lily • Cabomba (fanwort) • Brasenia (watershield) Ceratophyllales • Hornwort Austrobaileyales • Illicium (anise tree, star anise) Basal angiosperms - magnoliids Canellales • Drimys (winter's bark) • Tasmanian pepper Laurales • Bay laurel • Cinnamon • Avocado • Sassafras • Camphor tree • Calycanthus (sweetshrub, spicebush) • Lindera (spicebush, Benjamin bush) Magnoliales • Custard-apple • Pawpaw • guanábana (soursop) • Sugar-apple or sweetsop • Cherimoya • Magnolia • Tuliptree • Michelia • Nutmeg • Clove Piperales • Black pepper • Kava • Lizard’s tail • Aristolochia (birthwort, pipevine, Dutchman's pipe) • Asarum (wild ginger) Basal angiosperms - monocots Acorales -

Ethnopharmacology of Fruit Plants
molecules Review Ethnopharmacology of Fruit Plants: A Literature Review on the Toxicological, Phytochemical, Cultural Aspects, and a Mechanistic Approach to the Pharmacological Effects of Four Widely Used Species Aline T. de Carvalho 1, Marina M. Paes 1 , Mila S. Cunha 1, Gustavo C. Brandão 2, Ana M. Mapeli 3 , Vanessa C. Rescia 1 , Silvia A. Oesterreich 4 and Gustavo R. Villas-Boas 1,* 1 Research Group on Development of Pharmaceutical Products (P&DProFar), Center for Biological and Health Sciences, Federal University of Western Bahia, Rua Bertioga, 892, Morada Nobre II, Barreiras-BA CEP 47810-059, Brazil; [email protected] (A.T.d.C.); [email protected] (M.M.P.); [email protected] (M.S.C.); [email protected] (V.C.R.) 2 Physical Education Course, Center for Health Studies and Research (NEPSAU), Univel University Center, Cascavel-PR, Av. Tito Muffato, 2317, Santa Cruz, Cascavel-PR CEP 85806-080, Brazil; [email protected] 3 Research Group on Biomolecules and Catalyze, Center for Biological and Health Sciences, Federal University of Western Bahia, Rua Bertioga, 892, Morada Nobre II, Barreiras-BA CEP 47810-059, Brazil; [email protected] 4 Faculty of Health Sciences, Federal University of Grande Dourados, Dourados, Rodovia Dourados, Itahum Km 12, Cidade Universitaria, Caixa. postal 364, Dourados-MS CEP 79804-970, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-(77)-3614-3152 Academic Editors: Raffaele Pezzani and Sara Vitalini Received: 22 July 2020; Accepted: 31 July 2020; Published: 26 August 2020 Abstract: Fruit plants have been widely used by the population as a source of food, income and in the treatment of various diseases due to their nutritional and pharmacological properties. -

Spontaneous Poisoning by Talisia Esculenta in Cattle1 Jaianne K.A
Pesq. Vet. Bras. 39(12):949-953, December 2019 DOI: 10.1590/1678-5150-PVB-6362 Original Article Livestock Diseases ISSN 0100-736X (Print) ISSN 1678-5150 (Online) PVB-6362 LD Spontaneous poisoning by Talisia esculenta in cattle1 Jaianne K.A. Melo2, Gliére S.L. Soares2, Taciana R.R. Ramos3, Valdir M. Almeida4, Ana L.O. Nascimento5, Givaldo B. Silva Filho5, Hisadora A.S. Chaves5 and Fábio S. Mendonça5* ABSTRACT.- Melo J.K.A., Soares G.S.L., Ramos T.R.R., Almeida V.M., Nascimento A.L.O., Silva Filho G.B., Chaves H.A.S. & Mendonça F.S. 2019. Spontaneous poisoning by Talisia esculenta in cattle. Pesquisa Veterinária Brasileira 39(12):949-953. Laboratório de Diagnóstico Animal, Universidade Federal Rural de Pernambuco, Rua Dom Manoel de Medeiros s/n, Dois Irmãos, Recife, PE 52171-900, Brazil. E-mail: [email protected] Talisia esculenta, commonly known as pitombeira, is a tree which fruits are widely consumed by human beings in northeastern Brazil. The aim of this work is to describe the epidemiological, clinical and pathological aspects of two outbreaks of spontaneous poisoning by T. esculenta in cattle in the dry region of Pernambuco, northeastern Brazil. The cases occurred in the municipalities of São Bento do Una and Belo Jardim. From a total of 25 adult cattle, eight become sick after ingest T. esculenta leaves and fruits. Four cattle died until 72 hours after the spasms in the limbs, rigidity of the pelvic limbs with wide base stance position, ruminal atony firstand, clinicalwhen stressed, signs; which presented consisted falls in ataxia,and remained reluctance in toabnormal walk, tottering, positions. -

Illinois Native Plant Society 2019 Plant List Herbaceous Plants
Illinois Native Plant Society 2019 Plant List Plant Sale: Saturday, May 11, 9:00am – 1:00pm Illinois State Fairgrounds Commodity Pavilion (Across from Grandstand) Herbaceous Plants Scientific Name Common Name Description Growing Conditions Comment Dry to moist, Sun to part Blooms mid-late summer Butterfly, bee. Agastache foeniculum Anise Hyssop 2-4', Lavender to purple flowers shade AKA Blue Giant Hyssop Allium cernuum var. Moist to dry, Sun to part Nodding Onion 12-18", Showy white flowers Blooms mid summer Bee. Mammals avoid cernuum shade 18", White flowers after leaves die Allium tricoccum Ramp (Wild Leek) Moist, Shade Blooms summer Bee. Edible back Moist to dry, Part shade to Blooms late spring-early summer Aquilegia canadensis Red Columbine 30", Scarlet and yellow flowers shade Hummingbird, bee Moist to wet, Part to full Blooms late spring-early summer Showy red Arisaema dracontium Green Dragon 1-3', Narrow greenish spadix shade fruits. Mammals avoid 1-2', Green-purple spadix, striped Moist to wet, Part to full Blooms mid-late spring Showy red fruits. Arisaema triphyllum Jack-in-the-Pulpit inside shade Mammals avoid Wet to moist, Part shade to Blooms late spring-early summer Bee. AKA Aruncus dioicus Goatsbeard 2-4', White fluffy panicles in spring shade Brides Feathers Wet to moist, Light to full Blooms mid-late spring Mammals avoid. Asarum canadense Canadian Wildginger 6-12", Purplish brown flowers shade Attractive groundcover 3-5', White flowers with Moist, Dappled sun to part Blooms summer Monarch larval food. Bee, Asclepias exaltata Poke Milkweed purple/green tint shade butterfly. Uncommon Blooms mid-late summer Monarch larval Asclepias hirtella (Tall) Green Milkweed To 3', Showy white flowers Dry to moist, Sun food. -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Alvaradoa Amorphoides Germination at Low Water Potential and the Role of the Antioxidant System
Botanical Sciences 93 (2): -9, 205 PHYSIOLOGY DOI: 0.729/botsci.6 ALVARADOA AMORPHOIDES GERMINATION AT LOW WATER POTENTIAL AND THE ROLE OF THE ANTIOXIDANT SYSTEM VERÓNICA HERNÁNDEZ-PÉREZ1, JUDITH MÁRQUEZ-GUZMÁN2, SOBEIDA SÁNCHEZ-NIETO3 1, 4 AND ROCÍO CRUZ-ORTEGA 1Instituto de Ecología, Departamento de Ecología Funcional, Universidad Nacional Autónoma de México, México, D.F., Mexico 2Facultad de Ciencias, Laboratorio de Desarrollo de Plantas, Universidad Nacional Autónoma de México, México, D.F., Mexico 3Facultad de Química, Departamento de Bioquímica, Universidad Nacional Autónoma de México, México, D.F., Mexico 4Author for correspondence: Rocío Cruz-Ortega: [email protected] Abstract: Tropical dry forests are characterized by a high diversity of tree communities and extremely heterogeneous water avai- lability. The tree Alvaradoa amorphoides is a pioneer species of the tropical dry forest found in Xochicalco, Morelos, Mexico. To determine the water requirements for this species to germinate, we evaluated the seed germination rates under field and labo- ratory conditions. In the field, the seeds had an overall mean germination rate of 42%, but the rate varied between the different sites independent of the soil relative humidity and landscape. Alvaradoa amorphoides seeds exposed to a water potential of -0.5 MPa delayed germination, extending Phase II. At the -.0 and -.5 MPa water potentials, germination was inhibited by 80 and 00%, respectively, but the seeds remained viable. Although, the oxygen consumption did not differ between the treatments, the respiration profiles did not show the same triphasic curve as the control. The H2O2 and O2- levels were not significantly different in the seeds at the evaluated low-water potentials (-0.5 and -.0 MPa), nor were the catalase, superoxide dismutase, and gluta- thione reductase activity. -
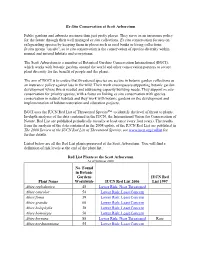
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

Evolutionary Consequences of Dioecy in Angiosperms: the Effects of Breeding System on Speciation and Extinction Rates
EVOLUTIONARY CONSEQUENCES OF DIOECY IN ANGIOSPERMS: THE EFFECTS OF BREEDING SYSTEM ON SPECIATION AND EXTINCTION RATES by JANA C. HEILBUTH B.Sc, Simon Fraser University, 1996 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA July 2001 © Jana Heilbuth, 2001 Wednesday, April 25, 2001 UBC Special Collections - Thesis Authorisation Form Page: 1 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. The University of British Columbia Vancouver, Canada http://www.library.ubc.ca/spcoll/thesauth.html ABSTRACT Dioecy, the breeding system with male and female function on separate individuals, may affect the ability of a lineage to avoid extinction or speciate. Dioecy is a rare breeding system among the angiosperms (approximately 6% of all flowering plants) while hermaphroditism (having male and female function present within each flower) is predominant. Dioecious angiosperms may be rare because the transitions to dioecy have been recent or because dioecious angiosperms experience decreased diversification rates (speciation minus extinction) compared to plants with other breeding systems. -

First Phylogeny of Bitterbush Family, Picramniaceae (Picramniales)
plants Article First Phylogeny of Bitterbush Family, Picramniaceae (Picramniales) Alexey Shipunov 1,*, Shyla Carr 1, Spencer Furniss 1, Kyle Pay 1 and José Rubens Pirani 2 1 Minot State University, Minot, ND 58707, USA; [email protected] (S.C.); [email protected] (S.F.); [email protected] (K.P.) 2 University of São Paulo, São Paulo 01000-000, Brazil; [email protected] * Correspondence: [email protected] Received: 17 December 2019; Accepted: 19 February 2020; Published: 21 February 2020 Abstract: Picramniaceae is the only member of Picramniales which is sister to the clade (Sapindales (Huerteales (Malvales, Brassicales))) in the rosidsmalvids. Not much is known about most aspects of their ecology, geography, and morphology. The family is restricted to American tropics. Picramniaceae representatives are rich in secondary metabolites; some species are known to be important for pharmaceutical purposes. Traditionally, Picramniaceae was classified as a subfamily of Simaroubaceae, but from 1995 on, it has been segregated containing two genera, Picramnia and Alvaradoa, with the recent addition of a third genus, Nothotalisia, described in 2011. Only a few species of the family have been the subject of DNA-related research, and fewer than half of the species have been included in morphological phylogenetic analyses. It is clear that Picramniaceae remains a largely under-researched plant group. Here we present the first molecular phylogenetic tree of the group, based on both chloroplast and nuclear markers, widely adopted in the plant DNA barcoding. The main findings are: The family and its genera are monophyletic and Picramnia is sister to two other genera; some clades corroborate previous assumptions of relationships made on a morphological or geographical basis, while most parts of the molecular topology suggest high levels of homoplasy in the morphological evolution of Picramnia.