Sand Dune Conservation, Management and Restoration Coastal Research Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Introducing Sand Dunes - Key Stage 3 and Key Stage 4
Introducing Sand Dunes - Key Stage 3 and Key Stage 4 Notes to accompany slide show – KS3 and KS4 Overview Description These are notes to accompany a PowerPoint presentation for Key Stage Three and Keys Stage 4 pupils. The PowerPoint along with the notes introduces the history and charac- teristics of sand dune systems, with emphasis on Woolacombe sand dunes and Braunton Burrows in North Devon. Time Approx. 30-40 minutes Curriculum Themes from this presentation can extend into studies of: KS3 Science – Interactions and interdependencies; Structure and Func- tions of Living Organisms; GCSE Science – Ecosystems – biodiversity, adaptations, positive and neg- ative impacts of humans on ecosystems; the Earth’s water resources. KS3 History – deepening students’ chronological understanding of histo- ry; local history study. GCSE History – ‘History Around Us’ KS3 Geography - understand how human and physical processes interact to influence and change landscapes, environments and the climate; phys- ical geography linking to soil, weather, climate and hydrology GCSE Geography – AQA Climate Change, Ecosystems; Edexcel 4.2 Physi- cal and human processes work together to create distinct UK landscapes. Introducing Sand Dunes - Keys Stage 3 Aims Give students an overview of the history of sand dunes in North Dev- on • Link the history of sand dunes to the present day characteristics of the dunes in terms of the physical landscape, biodiversity, land use, archaeology, industry and tourism. • Learning outcomes • Understand some of the chronological history of sand dunes in North Devon. • Understand some of the human and physical processes that have contributed to creating this unique landscape. • Understand what makes sand dunes have a high biodiversity and what that biodiversity profile looks like. -

High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project
High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project AEA Technology, Environment Contract: W/35/00632/00/00 For: The Department of Trade and Industry New & Renewable Energy Programme Report issued 30 August 2002 (Version with minor corrections 16 September 2002) Keith Hiscock, Harvey Tyler-Walters and Hugh Jones Reference: Hiscock, K., Tyler-Walters, H. & Jones, H. 2002. High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Report from the Marine Biological Association to The Department of Trade and Industry New & Renewable Energy Programme. (AEA Technology, Environment Contract: W/35/00632/00/00.) Correspondence: Dr. K. Hiscock, The Laboratory, Citadel Hill, Plymouth, PL1 2PB. [email protected] High level environmental screening study for offshore wind farm developments – marine habitats and species ii High level environmental screening study for offshore wind farm developments – marine habitats and species Title: High Level Environmental Screening Study for Offshore Wind Farm Developments – Marine Habitats and Species Project. Contract Report: W/35/00632/00/00. Client: Department of Trade and Industry (New & Renewable Energy Programme) Contract management: AEA Technology, Environment. Date of contract issue: 22/07/2002 Level of report issue: Final Confidentiality: Distribution at discretion of DTI before Consultation report published then no restriction. Distribution: Two copies and electronic file to DTI (Mr S. Payne, Offshore Renewables Planning). One copy to MBA library. Prepared by: Dr. K. Hiscock, Dr. H. Tyler-Walters & Hugh Jones Authorization: Project Director: Dr. Keith Hiscock Date: Signature: MBA Director: Prof. S. Hawkins Date: Signature: This report can be referred to as follows: Hiscock, K., Tyler-Walters, H. -

Auction - Sale 632: Golf Books by the Shelf 01/04/2018 11:00 AM PST
Auction - Sale 632: Golf Books by the Shelf 01/04/2018 11:00 AM PST Lot Title/Description Lot Title/Description 1 24 Golf Books 3 32 Golf Books Includes:Allen, Peter. Famous Fairways. London: Stanley Paul, Includes:Balata, Billy. Being The Ball. Phoenix, Arizona: B.T.B. 1968.Allison, Willie. The First Golf Review. London: Bonar Books, Entertainment, 2000.Beard, Frank. Shaving Strokes. New York: Grosset 1950.Alliss, Peter. A Golfer’s Travels. London: Boxtree, 1997.Alliss, & Dunlap, 1970.Canfield, Jack. Chicken Soup For The Golfer’s Soul: Peter. Bedside Golf. London: Collins, 1980.Alliss, Peter. More Bedside The 2nd Round. Florida: Health Communications, 2002.Canfield, Jack. Golf. London: Collins, 1984.Alliss, Peter. Yet More Bedside Golf. Chicken Soup For The Soul. Cos Cob, Connecticut: Chicken Soup For London: Collins, 1985.Ballesteros, Severiano. Seve. Connecticut: Golf The Soul Publishing, 2008.Canfield, Jack. Chicken Soup For The Soul Digest, 1982.Cotton, Henry. Thanks For The Game. London: Sidgwick & And Golf Digest Present THE GOLF BOOK. Cos Cob, Connecticut: Jackson, 1980.Crane, Malcolm. The Story Of Ladies’ Golf. London: Chicken Soup For The Soul Publishing, 2009.Canfield, Jack. Chicken Stanley Paul, 1991.Critchley, Bruce. Golf And All Its Glory. London: B B Soup For The Woman Golfer’s Soul. Florida: Health Communications, C Books, 1993.Follmer, Lucille. Your Sports Are Showing. : Pellegrini & 2007.Coyne, John. The Caddie Who Won The Masters. Oakland, Cudahy, 1949.Greene, Susan. Consider It Golf. SIGNED. Michigan: California: Peace Corps Writers Book, 2011.Ferguson, Allan Mcalister. Excel, 2000.Greene, Susan. Count On Golf. SIGNED. Michigan: Excel, Golf In Scotland. -

Antiproliferative Effects of Pancratium Maritimum Extracts on Normal and Cancerous Cells
IJMS Vol 43, No 1, January 2018 Original Article Antiproliferative Effects of Pancratium Maritimum Extracts on Normal and Cancerous Cells Ghaleb Tayoub1, PhD; Abstract Mohmad Al-Odat2, PhD; Amal Amer1, BSc; Background: Plants are an important natural source of Abdulmunim Aljapawe1, BSc; compounds used in cancer therapy. Pancratium maritimum Adnan Ekhtiar1,PhD contains potential anti-cancer agents such as alkaloids. In this study, we investigated the anti-proliferative effects of P. maritimum extracts on MDA-MB-231 human epithelial 1Department of Molecular Biology and Biotechnology, Atomic Energy adenocarcinoma cell line and on normal lymphocytes in vitro. Commission of Syria, Damascus, Syria; Methods: Leaves, flowers, roots, and bulbs of P. maritimum 2Department of Radiation Protection and Safety, Atomic Energy Commission of were collected and their contents were extracted and diluted to Syria, Damascus, Syria different concentrations that were applied on MDA-MB-231 cells and normal human lymphocytes in vitro for different intervals. Correspondence: Ghaleb Tayoub, PhD; Cells viability, proliferation, cell cycle distribution, apoptosis, Atomic Energy Commission of Syria, and growth were evaluated by flow cytometry and microscopy. P. O. Box: 6091, Damascus, Syria Parametric unpaired t-test was used to compare effects of plant Fax: +963 11 6112289 Tel: +963 11 2132580 extracts on treated cell cultures with untreated control cell Email: [email protected] cultures. IC50 was also calculated. Received: 3 September 2016 Results: P. maritimum extract had profound effects on Revised: 15 October 2016 Accepted: 13 November 2016 MDA-MB-321 cells. It inhibited cell proliferation in a dose- and time-dependent manner. The IC50 values were 0.039, 0.035, and 0.026 mg/ml after 48, 72, and 96 hours of treatment with 0.1 mg/ml concentration of bulb extract, respectively. -

Survey and Analysis of Vegetation and Hydrological Change in English Dune Slack Habitats
Natural England Commissioned Report NECR153 Survey and analysis of vegetation and hydrological change in English dune slack habitats First published 14 August 2014 www.naturalengland.org.uk Foreword Natural England commission a range of reports from external contractors to provide evidence and advice to assist us in delivering our duties. This work was conducted under a Memorandum of Agreement between Natural England and the Centre for Ecology and Hydrology and British Geological Survey, initiating a programme of linked vegetation and hydrological studies. Background Sand dune slacks also known as dune wetlands, In addition, for a limited number of 'key sites' in are a rare and threatened habitat in England. England to: The habitat is also of European significance and has suffered from limited research to date • Improve understanding of soil and geological because of the small sizes, rarity and conditions underpinning the dune sites. geographically peripheral location around the • Enhance long term water table monitoring. coast. The aim of this work is to improve the • Undertake fine detail water table monitoring of conservation status of this habitat through key dune slacks (annual cycle). increased understanding of dune • Produce ground terrain models of key dune ecohydrological functioning. slacks. The key elements of this work for all sites with • Quantify scrub evapotranspiration (Braunton dune wetlands are to: Burrows). • Develop 'conceptual models' of the • Produce an up-to-date inventory and hydrological functioning of key dune sites. description of dune wetland vegetation in England. Natural England will use the findings in a number of ways, including to information further • Provide information on soil conditions linked to research, report on the condition and status of vegetation data. -

Ron Arnst's HMG Course Collection Summaries
HISTORY MAKER GOLF Championship Golf Game • Course Summaries Course Collection ONE Blackjack GC, Las Vegas NV / based on TPC SUMMERLIN TPC Summerlin’s layout, carved from a magnificent swath of rugged desert terrain by renowned golf course architect Bobby Weed, provides a good reference for the Blackjack GC. TPC Summerlin’s lush bentgrass greens, numerous water features and an abundance of pine trees all contrast dramatically with undisturbed desert washes. The course features four closing holes that deliver top flight golf drama. The final charge begins with the par 4, 15th hole – a drivable par 4 that will temp most players. If the tee shot misses the green, an “up and down” birdie is possible, but not easy, due to the severely elevated and undulated green – which is surrounded by five bunkers that regularly attract stray tee shots. The 16th hole is a relatively downhill par-5 that is reachable with two good shots. The green is guarded by water short of the green, and bunkers beyond. Only a mid-iron will be necessary for the second shot, with a birdie almost a certainty. A challenging and un-nerving par-3, the 17th hole plays downhill with the green guarded closely by a lake on the left and by bunkers on the right. Par is good score and birdies are rare, should players need to make up ground. The 18th is a well-designed and strategic finishing hole which moves right to left off the tee. The green is protected on the left by a lake. An aggressive tee shot with the driver can leave the player just a short iron to a very deep green from front to back. -

2000-2009 Section History.Pub
A Chronicle of the Philadelphia Section PGA and its Members by Peter C. Trenham 2000 to 2009 2000 Jack Connelly was elected president of the PGA of America and John DiMarco won the New Jersey Open 2001 Terry Hatch won the stroke play and the match play tournaments at the PGA winter activities in Port St. Lucie 2002 The Section hosted the PGA of America national meeting at the Wyndham Franklin Plaza Hotel in Philadelphia 2003 Jim Furyk won the U.S. Open, Greg Farrow won the N.J. Open, Tom Carter won 3 times on the Nationwide Tour 2004 Pete Oakley won the Senior British Open 2005 Will Reilly was the PGA of America’s “ Junior Golf Leader” and Rich Steinmetz was on the PGA Cup Team 2006 Jim Furyk played on his fifth straight Ryder Cup Team, won the Vardon Trophy and two PGA Tour events 2007 In October the Philadelphia PGA and the Variety Club broke ground on the Variety Club’s 3-hole golf course 2008 Tom Carpus won the PGA of America’s Horton Smith Award and Hugh Reilly received the President Plaque 2009 Mark Sheftic finished second in the PGA Professional National Championship and played on the PGA Cup Team 2000 Jim Furyk won the Doral Open on the Doral Golf Resort’s Blue Course in the first week of March. The course nicknamed the “ Blue Monster” had been toughened in 1996 by adding 27 bunkers, which most of the play- ers didn’t care for. In 1999 the course had been reworked to its original Dick Wilson design, but now most of the players thought the course was too easy. -

Black's Guide to Devonshire
$PI|c>y » ^ EXETt R : STOI Lundrvl.^ I y. fCamelford x Ho Town 24j Tfe<n i/ lisbeard-- 9 5 =553 v 'Suuiland,ntjuUffl " < t,,, w;, #j A~ 15 g -- - •$3*^:y&« . Pui l,i<fkl-W>«? uoi- "'"/;< errtland I . V. ',,, {BabburomheBay 109 f ^Torquaylll • 4 TorBa,, x L > \ * Vj I N DEX MAP TO ACCOMPANY BLACKS GriDE T'i c Q V\ kk&et, ii £FC Sote . 77f/? numbers after the names refer to the page in GuidcBook where die- description is to be found.. Hack Edinburgh. BEQUEST OF REV. CANON SCADDING. D. D. TORONTO. 1901. BLACK'S GUIDE TO DEVONSHIRE. Digitized by the Internet Archive in 2010 with funding from University of Toronto http://www.archive.org/details/blacksguidetodevOOedin *&,* BLACK'S GUIDE TO DEVONSHIRE TENTH EDITION miti) fffaps an* Hlustrations ^ . P, EDINBURGH ADAM AND CHARLES BLACK 1879 CLUE INDEX TO THE CHIEF PLACES IN DEVONSHIRE. For General Index see Page 285. Axniinster, 160. Hfracombe, 152. Babbicombe, 109. Kent Hole, 113. Barnstaple, 209. Kingswear, 119. Berry Pomeroy, 269. Lydford, 226. Bideford, 147. Lynmouth, 155. Bridge-water, 277. Lynton, 156. Brixham, 115. Moreton Hampstead, 250. Buckfastleigh, 263. Xewton Abbot, 270. Bude Haven, 223. Okehampton, 203. Budleigh-Salterton, 170. Paignton, 114. Chudleigh, 268. Plymouth, 121. Cock's Tor, 248. Plympton, 143. Dartmoor, 242. Saltash, 142. Dartmouth, 117. Sidmouth, 99. Dart River, 116. Tamar, River, 273. ' Dawlish, 106. Taunton, 277. Devonport, 133. Tavistock, 230. Eddystone Lighthouse, 138. Tavy, 238. Exe, The, 190. Teignmouth, 107. Exeter, 173. Tiverton, 195. Exmoor Forest, 159. Torquay, 111. Exmouth, 101. Totnes, 260. Harewood House, 233. Ugbrooke, 10P. -

MN MI INM NM MI MI IM NM NINS ONNI M NINI ENIN NNE NINI IM NIM INEN NM a 1=11=11M1011•0111•111Flannimminiiimmomi
MN MI INM NM MI MI IM NM NINS ONNI M NINI ENIN NNE NINI IM NIM INEN NM a 1=11=11M1011•0111•111flaNNIMMINIIIMMOMI Ccarnt eint s Page Summary Author's Preface 6 Introduction 7 The effect of grazing on dune grassland communities 9 2.1 Grazing and the plant community 9 2.2 The development of sand dune vegetation 10 2.: The development of dune soils 11 2.4 Dune soil water levels 12 2.5 Yellow dune communities 13 2.6 Dune grassland communities 14 . 2.7 Dune slack communities 16 2.8 Dune heath communities 18 2.9 The control of plant species diversity 18 2.10 The impact of rabbits on sand dunes 21 2.11 The use of dunes for grazing farm animals 22 2.12 The increase of species diversity 22 " 3. Study methods 3.1- Selection of study sites 23 3.2 Survey methods used 28 3.3 Data from Scottish Survey 33 3.4 Other sources of information 36 Survey of British sand dunes 37 4.1 Seasonal changes 37 4.2 The present grazing situation 38 4.3 Yellow dune communities 42 4.4 Dune grassland communities 47 4.5 Damp slack communities 53 4.6 Wet slack communities 56 4.7 Dune heath communities 58 4.8 Species diversity 61 4.9 Regional trends 62 4.10 Grazing eperiments 64 Site assessments and recommendations 67 5.1 Introduction 67 5.7 North-east Scotland 67 5.3 South-east Scotland 70 5.4 North-east England 72 5.5 South-east England 74 5.6 South-west England and South Wales 77 5.7 North-west England and North Wales So 5.8 West Scotland 84 1 Assessment of grazing situations 89 6.1 .Introduction 89 6.2 Grazing by domestic animals 89 6.3 Grazing by rabbits 91 6.4 Grazing -
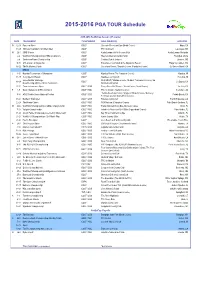
2015-16 PGA TOUR Schedule for RELEASE
2015-2016 PGA TOUR Schedule 2015-2016 FedExCup Season (47 events) DATE TOURNAMENT TV NETWORKS GOLF COURSE(S) LOCATION O 12-18 Frys.com Open GOLF Silverado Resort and Spa (North Course) Napa. CA 19-25 Shriners Hospitals for Children Open GOLF TPC Summerlin Las Vegas, NV N 26-1 CIMB Classic GOLF Kuala Lumpur Golf & Country Club Kuala Lumpur, Malaysia 2-8 World Golf Championships-HSBC Champions GOLF Sheshan International Golf Club Shanghai, China 2-8 Sanderson Farms Championship GOLF Country Club of Jackson Jackson, MS 9-15 OHL Classic at Mayakoba GOLF El Camaleon Golf Club at the Mayakoba Resort Playa del Carmen, MX 16-22 The McGladrey Classic GOLF Sea Island Resort (*Seaside Course, Plantation Course) St. Simons Island, GA BREAK J 4-10 Hyundai Tournament of Champions GOLF Kapalua Resort (The Plantation Course) Kapalua, HI 11-17 Sony Open in Hawaii GOLF Waialae Country Club Honolulu, HI CareerBuilder Challenge PGA WEST (*Stadium Course, Nicklaus Tournament Course); La 18-24 GOLF La Quinta, CA in partnership with the Clinton Foundation Quinta Country Club 25-31 Farmers Insurance Open GOLF / CBS Torrey Pines Golf Course (*South Course, North Course) La Jolla, CA F 1-7 Waste Management Phoenix Open GOLF / NBC TPC Scottsdale (Stadium Course) Scottsdale, AZ *Pebble Beach Golf Links, Spyglass Hill Golf Course, Monterey 8-14 AT&T Pebble Beach National Pro-Am GOLF / CBS Pebble Beach, CA Peninsula Country Club (Shore Course) 15-21 Northern Trust Open GOLF / CBS Riviera Country Club Pacific Palisades, CA 22-28 The Honda Classic GOLF / NBC PGA National -

Scotland for Golf – Ayrshire Area
Scotland for Golf – Ayrshire area Royal Troon Golf Club Founded in 1878, the club now has 3 courses and hosted the Open Championship on 8 occasions since 1923. The 2 x 18 hole courses are only open to visitors on Monday, Wednesday and Thursday. The Old Course One of the great links courses in Scotland, the Old Course is a challenging test of golfing ability. With the wind to contend with, and deep rough interspersed with gorse and broom, accurate shot making is essential. Players should make their scores on the outward nine, as the prevailing north-westerly wind can make the back nine extremely difficult. Portland Course Although a links course, the Portland is a little more sheltered than the Old Course and, of course, shorter. The holes meander through terrain filled with gorse and broom and has a generous helping of Par 3's, five in all. This is tempered with four Par 5's, all of which are on the back nine. Prestwick Golf Club Venue of the first Open Championship in 1860, hickory shafts and a gutty golf ball. Six of the Prestwick Golf Club hosted its 24th and final original greens are still played on today. Open Championship in 1925. The club also hosed 11 Amateur Championships between 1888 and 2001. A stone cairn to the west of the Clubhouse, marks the first tee of the original 12 hole course, on which the first Open was played. The 1st hole measured 578 yards to what is now the 16th green, where in 1870 Tom Morris Jr.