Views That Have Been Published in More Detail [6-13]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kentucky Derby, Flamingo Stakes, Florida Derby, Blue Grass Stakes, Preakness, Queen’S Plate 3RD Belmont Stakes
Northern Dancer 90th May 2, 1964 THE WINNER’S PEDIGREE AND CAREER HIGHLIGHTS Pharos Nearco Nogara Nearctic *Lady Angela Hyperion NORTHERN DANCER Sister Sarah Polynesian Bay Colt Native Dancer Geisha Natalma Almahmoud *Mahmoud Arbitrator YEAR AGE STS. 1ST 2ND 3RD EARNINGS 1963 2 9 7 2 0 $ 90,635 1964 3 9 7 0 2 $490,012 TOTALS 18 14 2 2 $580,647 At 2 Years WON Summer Stakes, Coronation Futurity, Carleton Stakes, Remsen Stakes 2ND Vandal Stakes, Cup and Saucer Stakes At 3 Years WON Kentucky Derby, Flamingo Stakes, Florida Derby, Blue Grass Stakes, Preakness, Queen’s Plate 3RD Belmont Stakes Horse Eq. Wt. PP 1/4 1/2 3/4 MILE STR. FIN. Jockey Owner Odds To $1 Northern Dancer b 126 7 7 2-1/2 6 hd 6 2 1 hd 1 2 1 nk W. Hartack Windfields Farm 3.40 Hill Rise 126 11 6 1-1/2 7 2-1/2 8 hd 4 hd 2 1-1/2 2 3-1/4 W. Shoemaker El Peco Ranch 1.40 The Scoundrel b 126 6 3 1/2 4 hd 3 1 2 1 3 2 3 no M. Ycaza R. C. Ellsworth 6.00 Roman Brother 126 12 9 2 9 1/2 9 2 6 2 4 1/2 4 nk W. Chambers Harbor View Farm 30.60 Quadrangle b 126 2 5 1 5 1-1/2 4 hd 5 1-1/2 5 1 5 3 R. Ussery Rokeby Stables 5.30 Mr. Brick 126 1 2 3 1 1/2 1 1/2 3 1 6 3 6 3/4 I. -

Shereif Hassan Mahmoud
Hydroclimate Changes to Arid regions subjected to Impact of climate change, human activities, and Large-scale climate patterns by Shereif Hassan Mahmoud A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Water Resources Engineering Department of Civil and Environmental Engineering University of Alberta © Shereif Hassan Mahmoud, 2020 Abstract In recent years, many regions worldwide have suffered from natural hazards related to the impact of human activities and climate change, such as floods and droughts, sea level rise, extreme weather events and an accelerated hydrological cycle. In Africa, the driest continent on Earth, climate change has led to more frequent occurrences of droughts of greater severity. Beside climate change, human activities have also incurred negative environmental impact which in turn has likely affected the climate at a wide range of temporal-spatial scales worldwide. For example, in the Middle East, floods of greater magnitude have been occurring more frequently in recent decades, which could be attributed partly to rapid urbanization or the effect of climate change, or both. In the Nile River basin (NRB), recurring droughts and increasing population have led to rising tension between competing users for water. Therefore, to develop more effective mitigation strategies against the potential impact of climate change, there is an urgent need to better understand changes to the hydrologic cycle of arid regions and linkage to regional climate change. The objectives of this dissertation are: 1) To investigate the potential implications of urbanization and climate change to the flood risk of Egypt and Saudi Arabia of arid climate in the Middle East. -

Mahmoud Saïd by Valérie Hess 74 Essay
Essay 73 Mahmoud Saïd By Valérie Hess 74 Essay Introduction referenced in art history courses in Western universities. For most art historians, the Bénézit dictionary, Nada Shabout’s lectures and seminars at the University initiated by Emmanuel Bénézit (1854-1920) in 1911, of North Texas are probably the most relevant to Saïd in is recognised as being probably the most important terms of subject and historical context. In other words, it and comprehensive reference dictionary for painters, seems that Mahmoud Saïd’s name, as most of his fellow sculptors, draughtsmen and engravers from all across Modern Egyptian artists, has simply been omitted from the world. Its full title in French is translated as ‘Critical the Western concept of the History of Art. and documentary dictionary of painters, sculptors, Paradoxically, several books and thesis written by locally draughtsmen and engravers from all times and from and internationally acclaimed academics, from Aimé all countries by a group of French and foreign expert Azar in 1961 to Esmat Dawastashy in 1997, from writers’ in the 1999 fourth edition published in fourteen Liliane Karnouk in 2005 to Nesma Atallah in 2010, volumes. This encyclopaedia of artists consists of 13,440 have rightfully identified Mahmoud Saïd as one of the pages for 175,000 names, yet nowhere does the name main pioneers of Modern Egyptian Art and arguably the ‘Mahmoud Saïd’ feature. The entries under the ‘SAI’ father of Modern Egyptian Painting. It therefore comes section are alphabetically listed as such: SAIA, Pietro with no surprise that one of the jewels of Alexandria, (1779-1833); SAÏD (See also LÉVY Alphonse Jacques); the artist’s native city, is a museum entirely dedicated to SAÏD, Anne (1914-1995); SAIDI, Aboul Ghasem (b. -

Lady Eli Retired, to Be Bred to War Front
WEDNESDAY, JANUARY 17, 2018 LADY ELI RETIRED, TO BE BREEDERS= CUP EYES FUTURE HOST SITES, POSSIBLE NEW RACE by Perry Lefko BRED TO WAR FRONT The Board of the Breeders' Cup World Championships will meet later this month to talk about tracks that could potentially host the event from 2019-22 and also discuss the possibility of adding another race. Fred Hertrich III, voted in as the Breeders' Cup Chairman last September, told the TDN that six tracks have expressed tentative interest in hosting the event after this year's Breeders' Cup World Championships at Churchill Downs, November 2-3. All six have been sent letters of tentative interest. Hertrich said when the Breeders' Cup Ltd. hears back from the tracks, it will send out contracts outlining all the details, dates and demands to be considered and see how many respond. Cont. p5 Lady Eli & Irad Ortiz, Jr. | Sarah K Andrew IN TDN EUROPE TODAY by Bill Finley After considering the possibility of racing their star mare this AN ENDURING DREAM FOR CHEVELEY PARK STUD year, co-owners Jay Hanley and Sol Kumin, partners in the Sheep Emma Berry speaks with Chris Richardson regarding Pond Partners stable, have decided to retire Lady Eli (Divine Cheveley Park Stud’s new stallion acquisition Ulysses (Ire) Park--Sacre Coeur, by Saint Ballado). She will be bred to War (Galileo {Ire}). Click or tap here to go straight to TDN Europe. Front and then sold at the 2018 Keeneland November Breeding Stock sale as part John Sikura=s Hill >n= Dale consignment. Following a seventh-place finish in the GI Breeders= Cup Filly & Mare Turf, Lady Eli was entered in the 2017 Keeneland sale, but had to be withdrawn when coming out of the race with lacerations and a puncture wound, which required rest and treatment. -

Sunday, March 1, 2020 Today in the Tdn Ete Indien All The
SUNDAY, MARCH 1, 2020 TODAY IN THE TDN ETE INDIEN ALL THE WAY IN FOUNTAIN OF YOUTH MR FREEZE DOMINATES GULFSTREAM PARK MILE TONALIST=S SHAPE STILL PERFECT AFTER DAVONA DALE PEGASUS TURF WINNER ADDS TO BANKROLL BODEMEISTER FILLY STEALS ANOTHER ONE AT GP IN TDN EUROPE SPEIGHTSTOWN=S FULL FLAT TAKES THE SAUDI DERBY FRANKEL EXACTA IN THE LONGINES TURF CUP ATTENDANCE RESTRICTED AT THREE FRENCH MEETINGS Click or tap here to go straight to TDN Europe/International PUBLISHER & CEO Sue Morris Finley @suefinley [email protected] SENIOR VICE PRESIDENT Gary King @garykingTDN [email protected] EDITORIAL [email protected] Editor-in-Chief Jessica Martini @JessMartiniTDN Managing Editor Sunday, March 1, 2020 Alan Carasso @EquinealTDN Senior Editor Steve Sherack @SteveSherackTDN Racing Editor Brian DiDonato @BDiDonatoTDN Deputy Editor Christie DeBernardis @CDeBernardisTDN Associate Editors Christina Bossinakis @CBossTDN Joe Bianca @JBiancaTDN News and Features Editor In Memoriam: Ben Massam (1988-2019) ADVERTISING [email protected] Director of Advertising Alycia Borer Advertising Manager Lia Best Advertising Designer Amanda Crelin Advertising Assistant/Dir. Of Distribution Rachel McCaffrey Advertising Assistants Amie Newcomb Saudi Cup coverage continues on page 3. | Jockey Club of Saudi Arabia/Mahmoud Khaled Kristen Lomasson Photographer/Photo Editor 70 HOURS IN RIYADH Sarah K. Andrew @SarahKAndrew 5 [email protected] Editor-in-Chief Jessica Martini shares her impressions of Saudi Arabia and King Abdulaziz racetrack. Social Media Strategist Justina Severni Director of Customer Service Vicki Forbes ETE INDIEN GOES GATE TO WIRE IN FOY 9 [email protected] Patrick Biancone trainee Ete Indien (Summer Front) rushed to the front from his wide draw and never Marketing Manager looked back to take Saturday’s GII Fasig-Tipton Fountain Alayna Cullen @AlaynaCullen of Youth S. -

BUTTERS FAMILY There Were 4 Brothers Who Attended
BUTTERS FAMILY There were 4 brothers who attended Framlingham between 1888 and 1905 and a couple of them were very prominent racehorse trainers. JOSEPH ARTHUR “FRANK” BUTTERS (1888-95) Frank Butters was Champion Trainer 8 times between 1927 and 1949. In addition to training the winners of 15 Classics in England he also trained Irish Derby winners Turkhan, Nathoo and Hindostan and Prix de L'Arc de Triomphe winner Migoli for the Aga Khan III. Frank Butters was born in Austria, where his father Joseph rode and trained, and became a successful trainer there himself. During the 1914-18 war he was nominally interned, then trained in Italy before being given a 4 year contract as private trainer to the 17th Earl of Derby at Stanley House, Newmarket, becoming leading trainer in his first year there, 1927. When Lord Derby withdrew from racing for economic reasons, Butters leased the Fitzroy Stables in Newmarket as a public trainer. Here he trained for Mr A W Gordon and, later, the Aga Khan III and the 5th Earl of Durham. He considered Bahram to be the best horse he ever trained. Important successes: 2000 Guineas 1000 Guineas Derby Bahram 1935 (T) Fair Isle 1930 (T) Bahram 1935 (T) Mahmoud 1936 (T) Oaks St Leger Other major race(s) Beam 1927 (T) Fairway 1928 (T) (featuring horses in this database) Toboggan 1928 (T) Firdaussi 1932 (T) Princess of Wales's Stakes Colorado 1927 (T) Udaipur 1932 (T) Bahram 1935 (T) Eclipse Stakes Colorado 1927 (T) Light Brocade 1934 (T) Turkhan 1940 (T) Eclipse Stakes Fairway 1928 (T) Steady Aim 1946 (T) Tehran 1944 (T) Champion Stakes Fairway 1928 (T) Masaka 1948 (T) Champion Stakes Fairway 1929 (T) Champion Stakes Umidwar 1934 (T) Jockey Club Stakes Umidwar 1934 (T) Dewhurst Stakes, Newmarket Bala Hissar 1935 (T) LTCOLONEL JAMES WAUGH BUTTERS OBE (1889-1904) Unlike Frank, he doesn’t appear to have been involved at all in horse racing. -

The Interface of Religious and Political Conflict in Egyptian Theatre
The Interface of Religious and Political Conflict in Egyptian Theatre Dissertation Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Amany Youssef Seleem, Stage Directing Diploma Graduate Program in Theatre The Ohio State University 2013 Dissertation Committee: Lesley Ferris, Advisor Nena Couch Beth Kattelman Copyright by Amany Seleem 2013 Abstract Using religion to achieve political power is a thematic subject used by a number of Egyptian playwrights. This dissertation documents and analyzes eleven plays by five prominent Egyptian playwrights: Tawfiq Al-Hakim (1898- 1987), Ali Ahmed Bakathir (1910- 1969), Samir Sarhan (1938- 2006), Mohamed Abul Ela Al-Salamouni (1941- ), and Mohamed Salmawi (1945- ). Through their plays they call attention to the dangers of blind obedience. The primary methodological approach will be a close literary analysis grounded in historical considerations underscored by a chronology of Egyptian leadership. Thus the interface of religious conflict and politics is linked to the four heads of government under which the playwrights wrote their works: the eras of King Farouk I (1920-1965), President Gamal Abdel Nasser (1918-1970), President Anwar Sadat (1918-1981), and President Hosni Mubarak (1928- ). While this study ends with Mubarak’s regime, it briefly considers the way in which such conflict ended in the recent reunion between religion and politics with the election of Mohamed Morsi, a member of the Muslim Brotherhood, as president following the Egyptian Revolution of 2011. This research also investigates how these scripts were written— particularly in terms of their adaptation from existing canonical work or historical events and the use of metaphor—and how they were staged. -

Novel Cavities in Ultrafast Vertical External Cavity Surface Emitting Lasers for High Power Harmonic Generation
Novel Cavities in Ultrafast Vertical External Cavity Surface Emitting Lasers for High Power Harmonic Generation Item Type text; Electronic Dissertation Authors Meyer, Jason Timothy Citation Meyer, Jason Timothy. (2021). Novel Cavities in Ultrafast Vertical External Cavity Surface Emitting Lasers for High Power Harmonic Generation (Doctoral dissertation, University of Arizona, Tucson, USA). Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction, presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 06/10/2021 03:26:45 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/660288 NOVEL CAVITIES IN ULTRAFAST VERTICAL EXTERNAL CAVITY SURFACE EMITTING LASERS FOR HIGH POWER HARMONIC GENERATION By Jason T. Meyer Copyright © Jason T. Meyer 2021 A Dissertation Submitted to the Faculty of the JAMES C. WYANT COLLEGE OF OPTICAL SCIENCES In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2021 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Jason Timothy Meyer, titled Novel Cavities in Ultrafast Vertical External Cavity Surface Emitting Lasers for High Power Harmonic Generation and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy. _________________________________________________________________ Date: ____________5/5/21 Professor Mahmoud Fallahi _________________________________________________________________ Date: ____________5/5/21 Professor Stanley K. -
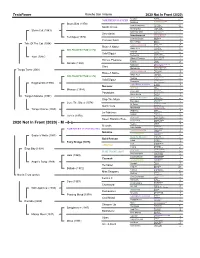
2020 Not in Front (2020)
TesioPower Rancho San Antonio 2020 Not In Front (2020) Nearctic Nearco 4 NORTHERN DANCER Lady Angela 14-c Natalma NATIVE DANCER 5 Storm Bird (1978) ALMAHMOUD 2 New Providence Bull Page 4-m South Ocean Fair Colleen 9-d Shining Sun Chop Chop 2 Storm Cat (1983) Solar Display 4-j BOLD RULER NASRULLAH 9 Secretariat Miss Disco 8 Somethingroyal PRINCEQUILLO 1 Terlingua (1976) IMPERATRICE 2-s Crimson Satan Spy Song 2 Crimson Saint Papila 26 Bolero Rose Bolero 6 Tale Of The Cat (1994) First Rose 8 NATIVE DANCER Polynesian 14 Raise A Native Geisha 5-f Raise You Case Ace 1-k MR PROSPECTOR (1970) Lady Glory 8-f Nashua NASRULLAH 9 Gold Digger Segula 3-m Sequence Count Fleet 6 Yarn (1987) Miss Dogwood 13 What A Pleasure BOLD RULER 8 Honest Pleasure Grey Flight 5 Tularia Tulyar 22 Narrate (1980) Suntop 11-f Nijinsky II NORTHERN DANCER 2 State Flaming Page 8 Monarchy PRINCEQUILLO 1 Tango Tales (2001) Knight's Daughter 2-f NATIVE DANCER Polynesian 14 Raise A Native Geisha 5-f Raise You Case Ace 1-k MR PROSPECTOR (1970) Lady Glory 8-f Nashua NASRULLAH 9 Gold Digger Segula 3-m Sequence Count Fleet 6 Kingmambo (1990) Miss Dogwood 13 NORTHERN DANCER Nearctic 14 Nureyev Natalma 2 SPECIAL Forli 3 Miesque (1984) Thong 5 Prove Out Graustark 4 Pasadoble Equal Venture 4 Santa Quilla Sanctus 16 Tango's Mambo (1997) Neriad 20 HAIL TO REASON TURN-TO 1 Stop The Music Nothirdchance 4-n Bebopper TOM FOOL 3 Cure The Blues (1978) Bebop II 11 Dr Fager Rough 'n Tumble 1 Quick Cure Aspidistra 1-r Speedwell BOLD RULER 8 Tango Charlie (1989) IMPERATRICE 2-s Wild Risk Rialto -

Competitive Bidding As Book 3 Opens at Keeneland
MONDAY, SEPTEMBER 16, 2019 COMPETITIVE BIDDING CLEMENT ‘INVADER’ PUNCHES BREEDERS’ CUP TICKET WITH SUMMER SCORE AS BOOK 3 OPENS Decorated Invader (Declaration of War), bet hard throughout, backed up his support and overcame a slow pace to capture the AT KEENELAND GI Summer S. Sunday at Woodbine, a “Win and You’re In” race for the GI Breeders’ Cup Juvenile Turf. Showing a strong late kick to be second debuting July 13 at Saratoga, the $200,000 Keeneland September grad won geared down there next out Aug. 10 and was crushed down to even- money from a 9-2 morning line early in the wagering here before drifting up to fractional second favoritism by post. Away a step slowly, the West Point Thoroughbreds colorbearer tugged his way up a few spots into seventh as huge longshot Cadet Connelly (Grey Swallow {Ire}) went on with it through pedestrian splits of :24.26 and :48.43 over yielding turf. Cont. p9 IN TDN EUROPE TODAY PINATUBO ELECTRIC IN THE NATIONAL Session-topping Hip 1385 in the ring | Keeneland Godolphin’s Pinatubo (Ire) (Shamardal) justified all the hype with a nine-length romp in The Curragh’s G1 National S. Click or tap here to go straight to TDN Europe. by Jessica Martini LEXINGTON, KY - The Keeneland September Yearling Sale’s Book 3 section opened with a day of competitive bidding Sunday in Lexington as 256 yearlings changed hands for a total of $30,025,000. The sixth session average was $117,285 and the median was $85,000. Of the 366 horses offered, 110 failed to reach their reserves for a buy-back rate of 30.05%. -

Airoforce Stalllion Analysis 9-9-19
STALLION ANALYSIS DATE: 9/8/19 (Colonel John - Chocolate Pop, by Cuvee) IRON MAIDENS THOROUGHBREDS [email protected] IRON MAIDENS THOROUGHBREDS Pedigree Consultant SECTION I STALLION AND IMMEDIATE FAMILY http://www.ironmaidensthoroughbreds.com ♦ 1 ♦ [email protected] ♦ 772-475-9798 IRON MAIDENS THOROUGHBREDS Pedigree Consultant AIROFORCE 2019 STALLION MATING ANALYSIS AIROFORCE (Colonel John - Chocolate Pop, by Cuvee) 2013 Gray/Roan 16.2 Hands Record: 12-3-3-0 ($679,130) Section I: STALLION AND IMMEDIATE FAMILY Airoforce is Colonel John’s most accomplished son. He has the long, lean elegance of a classic Thoroughbred, reminiscent of his sire Colonel John and second damsire, Canadian Champion With Approval, yet Airoforce received precocious speed from his damsire Cuvee. Airoforce was victorious at distances ranging between 6 1/2-furlongs and 1 1/16-miles. Although he competed mainly on turf, Airoforce won a key stake over dirt. His immediate pedigree has dirt and turf attributes. A magnificent looking $350,00 OBS April sales purchase campaigned by John Oxley, Airoforce flashed precociousness and speed in his debut, drawing away to win a six-furlong turf sprint by 3 1/4-lengths in a sharp 1:10.34, just a second off the Kentucky Downs track record. Airoforce dominated the Dixiana Bourbon Stakes (G3) by 2 1/2-lengths over a full field of fourteen over the Keeneland turf, including Camelot Kitten, Surgical Strike. Airoforce faced a full field of high-class youngsters as the favorite in the 2015 Breeders’ Cup Juvenile Turf (G1). and missed the victory by a neck, after racing in traffic and being distracted in the stretch. -

FOR the HYDROPONICS FARM in TANNUR – WADI Al HESSA
THE WORLD BANK - ENVIRONMENTAL AND SOCIAL COMMITMENT PLAN (ESCP) The Hashemite Kingdom of Jordan ENVIRONMENTAL AND SOCIAL IMPACT ASSESSMENT Public Disclosure Authorized (ESIA final Report) FOR THE HYDROPONICS FARM IN TANNUR – WADI Al HESSA, JORDAN Public Disclosure Authorized Public Disclosure Authorized Submitted to: Ministry of Agriculture Prepared by: Dr. Mahmoud Rabai Dr. Mahmoud Freihat Eng. Mahmoud Abu Jamous Eng. Hazem Al-Smadi Eng. Hussein Al-Qatameen Eng. Nada Freihat Public Disclosure Authorized Eng. Hussin Hawileh Amman-Jordan December, 2019 1 | P a g e THE WORLD BANK - ENVIRONMENTAL AND SOCIAL COMMITMENT PLAN (ESCP) TEMPLATE Version 2, July 2019 Government of Jordan/ Ministry of Agriculture “Exploring high-value, socially-inclusive, and water-efficient agriculture project in Jordan” ENVIRONMENTAL and SOCIAL COMMITMENT PLAN (ESCP) March 2020 2 | P a g e THE WORLD BANK - ENVIRONMENTAL AND SOCIAL COMMITMENT PLAN (ESCP) ENVIRONMENTAL AND SOCIAL COMMITMENT PLAN 1. 1. Government of Jordan/Ministry of Agriculture will implement Exploring high-value, socially-inclusive, and water-efficient agriculture project in Jordan”, with the involvement of the following Ministries/agencies/units: Ministry of Finance (MoF), Ministry of Planing and International Cooperation (MOBIC), Jordan Cooperative Corporation (JCC,)Private sector, farmers, local beneficiaries and NGOs. The International Bank for Reconstruction and Development/International Development Association] (hereinafter the [Bank/the Association) is providing financing for the Project. 2. Ministry of Agriculture will implement material measures and actions so that the Project is implemented in accordance with the Environmental and Social Standards (ESSs). This Environmental and Social Commitment Plan (ESCP) sets out material measures and actions, any specific documents or plans, as well as the timing for each of these.