NEW--Npf-MASTER LIST.Xlsx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
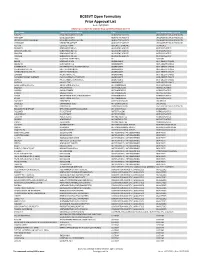
BCBSVT Open Formulary Prior Approval List
BCBSVT Open Formulary Prior Approval List As of: 10/27/2020 Helpful Tip: To search for a specific drug, use the find feature (Ctrl + F) Trade Name Chemical/Biological Name Class Prior Authorization Program FUSILEV LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS KHAPZORY LEVOLEUCOVORIN ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS LEVOLEUCOVORIN CALCIUM LEVOLEUCOVORIN CALCIUM ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS VISTOGARD URIDINE TRIACETATE ADJUNCTIVE AGENTS UNCLASSIFIED DRUG PRODUCTS ACTHAR CORTICOTROPIN ADRENAL HORMONES HORMONES BELRAPZO BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDAMUSTINE HCL BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS BENDEKA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS TREANDA BENDAMUSTINE HCL ALKYLATING AGENTS ANTINEOPLASTICS DAW (DISPENSE AS WRITTEN) ALL CUSTOM BELVIQ LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS BELVIQ XR LORCASERIN HCL ANOREXIANTS ANTI‐OBESITY DRUGS CONTRAVE ER NALTREXONE HCL/BUPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS DIETHYLPROPION HCL ER DIETHYLPROPION HCL ANOREXIANTS ANTI‐OBESITY DRUGS LOMAIRA PHENTERMINE HCL ANOREXIANTS ANTI‐OBESITY DRUGS PHENDIMETRAZINE TARTRATE PHENDIMETRAZINE TARTRATE ANOREXIANTS ANTI‐OBESITY DRUGS QSYMIA PHENTERMINE/TOPIRAMATE ANOREXIANTS ANTI‐OBESITY DRUGS SAXENDA LIRAGLUTIDE ANOREXIANTS ANTI‐OBESITY DRUGS ABIRATERONE ACETATE ABIRATERONE ACETATE ANTIANDROGENS ANTINEOPLASTICS ERLEADA APALUTAMIDE ANTIANDROGENS ANTINEOPLASTICS NUBEQA DAROLUTAMIDE ANTIANDROGENS -

2018-2019 Targeted Medication Safety Best Practices for Hospitals
2018-2019 Targeted Medication Safety Best Practices for Hospitals The purpose of the Targeted Medication Safety Best Practices for Hospitals is to identify, inspire, and mobilize widespread, national adoption of consensus-based best practices for specific medication safety issues that continue to cause fatal and harmful errors in patients, despite repeated warnings in ISMP publications. Hospitals can focus their medication safety efforts over the next 2 years on these best practices, which are realistic and have been successfully adopted by numerous organizations. While targeted for the hospital-based setting, some best practices may be applicable to other healthcare settings. The Targeted Medication Safety Best Practices for Hospitals have been reviewed by an external expert advisory panel and approved by the ISMP Board of Trustees. Related issues of the ISMP Medication Safety Alert! are referenced after each best practice. ISMP encourages hospitals that have not implemented the 2016-2017 Targeted Medication Safety Best Practices for Hospitals to do so as a priority, while implementing the 2018-2019 best practices. Organizations need to focus on previous best practices 2, 3, 9 and 11 since these have the lowest implementation rate. Two of the 2016-2017 Targeted Medication Safety Best Practices for Hospitals (number 4 and 7) have been revised for 2018-2019. Best practices number 12 through 14 are new for 2018-2019. www.ismp.org BEST PRACTICE 1: Dispense vinCRIStine (and other vinca alkaloids) in a minibag of a compatible solution and not in a syringe. Rationale: Related ISMP Medication The goal of this best practice is to ensure that vinca alkaloids are Safety Alerts!: administered by the intravenous route only. -

WHO Drug Information Vol 22, No
WHO Drug Information Vol 22, No. 1, 2008 World Health Organization WHO Drug Information Contents Challenges in Biotherapeutics Miglustat: withdrawal by manufacturer 21 Regulatory pathways for biosimilar Voluntary withdrawal of clobutinol cough products 3 syrup 22 Pharmacovigilance Focus Current Topics WHO Programme for International Drug Proposed harmonized requirements: Monitoring: annual meeting 6 licensing vaccines in the Americas 23 Sixteen types of counterfeit artesunate Safety and Efficacy Issues circulating in South-east Asia 24 Eastern Mediterranean Ministers tackle Recall of heparin products extended 10 high medicines prices 24 Contaminated heparin products recalled 10 DacartTM development terminated and LapdapTM recalled 11 ATC/DDD Classification Varenicline and suicide attempts 11 ATC/DDD Classification (temporary) 26 Norelgestromin-ethynil estradiol: infarction ATC/DDD Classification (final) 28 and thromboembolism 12 Emerging cardiovascular concerns with Consultation Document rosiglitazone 12 Disclosure of transdermal patches 13 International Pharmacopoeia Statement on safety of HPV vaccine 13 Cycloserine 30 IVIG: myocardial infarction, stroke and Cycloserine capsules 33 thrombosis 14 Erythropoietins: lower haemoglobin levels 15 Recent Publications, Erythropoietin-stimulating agents 15 Pregabalin: hypersensitivity reactions 16 Information and Events Cefepime: increased mortality? 16 Assessing the quality of herbal medicines: Mycophenolic acid: pregnancy loss and contaminants and residues 36 congenital malformation 17 Launch -

Human Chorionic Gonadotropin (HCG), a Polypeptide Hormone Produced by the Human
45792G/Revised: April 2011 CHORIONIC GONADOTROPIN FOR INJECTION, USP DESCRIPTION: Human chorionic gonadotropin (HCG), a polypeptide hormone produced by the human placenta, is composed of an alpha and a beta sub-unit. The alpha sub-unit is essentially identical to the alpha sub-units of the human pituitary gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), as well as to the alpha sub-unit of human thyroid-stimulating hormone (TSH). The beta sub-units of these hormones differ in amino acid sequence. Chorionic gonadotropin is obtained from the human pregnancy urine. It is standardized by a biological assay procedure. Chorionic Gonadotropin for Injection, USP is available in multiple dose vials containing 10,000 USP Units with accompanying Bacteriostatic Water for Injection for reconstitution. When reconstituted with 10 mL of the accompanying diluent each vial contains: Chorionic gonadotropin 10,000 Units Mannitol 100 mg Benzyl alcohol 0.9% Water for Injection q.s. Buffered with dibasic sodium phosphate and monobasic sodium phosphate. Hydrochloric acid and/or sodium hydroxide may have been used for pH adjustment (6.0 Reference ID: 2933198 8.0). Nitrogen gas is used in the freeze drying process. CLINICAL PHARMACOLOGY: The action of HCG is virtually identical to that of pituitary LH, although HCG appears to have a small degree of FSH activity as well. It stimulates production of gonadal steroid hormones by stimulating the interstitial cells (Leydig cells) of the testis to produce androgens and the corpus luteum of the ovary to produce progesterone. Androgen stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present. -

Gonadotropin and Testosterone Measurements After
Pediat. Res. 10: 46-51 (1976) Estradiol puberty estrogen sexual differentiation gonadotropins testosterone maturation Gonadotropin and Testosterone Measurements after Estrogen Administration to Adult Men, Prepubertal and Pubertal Boys, and Men with Hypogonadotropism: Evidence for Maturation of Positive Feedback in the Male HOWARD E. KULIN"" AND EDWARD 0. REITER Reproduction Research Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA Extract MATERIALS AND METHODS Nineteen male subjects were given five daily injections of SUBJECTS 17~-estradiol and circulating levels of estradiol (E 2 ), testosterone (T), and gonadotropins were determined by radioimmunoassay Seven normal, adult men (ages 20-22) and one male (age 21) before, during, and after the steroid course. Peak levels of E 2 with the syndrome of vanishing testes (I) were hospitalized for attained during the 5 days of treatment ranged from 173-577 pg/ml. study at the Clinical Center of the National Institutes of Health. Four of seven normal adult men and one castrate man demonstrated Each patient was given five daily intramuscular injections of IO or suppression of follicle-stimulating hormone ( FSH) and luteinizing 15 ,ug/kg body weight of 17~-estradiol (E2 ) (20) and blood samples hormone ( LH) with a subsequent rise in LH ( positive feedback) were obtained every 12-24 hr before, during, and after the estrogen while E 2 levels remained elevated. A rise in T was associated with course (see Table I). the LH increment in the four normal men. Nine pre-, early, or Nine endocrinologically normal boys between the ages of 7 and midpubertal boys and two men with hypogonadotropic hypogo 18 were studied in the course of evaluation for short stature, nadism displayed only gonadotropin suppression after E 2 adminis precocious puberty, or delayed adolescence. -

Chorionic Gonadotropin Human (C0684)
Chorionic gonadotropin human Product Number C 0684 Storage Temperature -0 °C Product Description When hCG was used in combination with recombinant CAS Number: 9002-61-3 interferon-γ, there was a significant cooperative pI = 2.951 induction of nitric oxide synthesis (iNOS) in a dose- Extinction Coefficient: E1% = 3.88 (278nm)2 dependent manner in mouse peritoneal macrophages Synonym: Choriogonin, hCG suggesting that hCG may provide a second signal for synergistic induction of NO synthesis.9 The molecular weight is approximately 37.9 kDa (with approximately 31% carbohydrate by weight). The Precautions and Disclaimer theoretical molecular weight is 37.9 kDa based on the For Laboratory Use Only. Not for drug, household or native form, which contains 2 subunits. The α subunit other uses. has a molecular weight of 14.9 kDa of which approximately 10.2 kDa is for the polypeptide and Preparation Instructions approximately 4.7 kDa for the carbohydrate. The hCG is soluble in water and aqueous buffers such β subunit has a molecular weight of 23 kDa of which phosphate buffer. hCG is also soluble in aqueous approximately 16.0 kDa is for the polypeptide and glycerol and glycols and is insoluble in ethanol.1 approximately 7.0 kDa is for the carbohydrate.3,4,5 Solutions should be sterile filtered and not autoclaved. Product Number C 0684 is sterile filtered and contains Storage/Stability approximately 1,000 I.U. per vial. Dilute aqueous solutions undergo rapid loss of activity when stored frozen, or heated, or if excess acid or hCG is a glycoprotein hormone produced by the base is added. -

The Effectiveness of Correcting Abnormal Metabolic Profiles
Received: 25 April 2019 Revised: 17 June 2019 Accepted: 19 June 2019 DOI: 10.1002/jimd.12139 ORIGINAL ARTICLE The effectiveness of correcting abnormal metabolic profiles Peter Theodore Clayton UCL Great Ormond Street Institute of Child Health, London, UK Abstract Inborn errors of metabolism cause disease because of accumulation of a metabolite Correspondence before the blocked step or deficiency of an essential metabolite downstream of the Peter Clayton, UCL Great Ormond Street Institute of Child Health, London, UK. block. Treatments can be directed at reducing the levels of a toxic metabolite or Email: [email protected] correcting a metabolite deficiency. Many disorders have been treated successfully first in a single patient because we can measure the metabolites and adjust treat- Communicating Editor: Sander M. Houten ment to get them as close as possible to the normal range. Examples are drawn from Komrower's description of treatment of homocystinuria and the author's trials 5 of treatment in bile acid synthesis disorders (3β-hydroxy-Δ -C27-steroid dehydro- genase deficiency and Δ4-3-oxosteroid 5β-reductase deficiency), neurotransmitter amine disorders (aromatic L-amino acid decarboxylase [AADC] and tyrosine hydroxylase deficiencies), and vitamin B6 disorders (pyridox(am)ine phosphate oxidase deficiency and pyridoxine-dependent epilepsy [ALDH7A1 deficiency]). Sometimes follow-up shows there are milder and more severe forms of the disease and even variable clinical manifestations but by measuring the metabolites we can adjust the treatment to get the metabolites into the normal range. Biochemical mea- surements are not subject to placebo effects and will also show if the disorder is improving spontaneously. -

Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry
Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document should be submitted within 90 days of publication in the Federal Register of the notice announcing the availability of the draft guidance. Submit electronic comments to https://www.regulations.gov. Submit written comments to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD 20852. All comments should be identified with the docket number listed in the notice of availability that publishes in the Federal Register. For questions regarding this draft document, contact Elaine Chang at 240-402-2628. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) July 2019 Clinical/Medical Advanced Prostate Cancer: Developing Gonadotropin- Releasing Hormone Analogues Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353; Email: [email protected] https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation -

WSAVA List of Essential Medicines for Cats and Dogs
The World Small Animal Veterinary Association (WSAVA) List of Essential Medicines for Cats and Dogs Version 1; January 20th, 2020 Members of the WSAVA Therapeutic Guidelines Group (TGG) Steagall PV, Pelligand L, Page SW, Bourgeois M, Weese S, Manigot G, Dublin D, Ferreira JP, Guardabassi L © 2020 WSAVA All Rights Reserved Contents Background ................................................................................................................................... 2 Definition ...................................................................................................................................... 2 Using the List of Essential Medicines ............................................................................................ 2 Criteria for selection of essential medicines ................................................................................. 3 Anaesthetic, analgesic, sedative and emergency drugs ............................................................... 4 Antimicrobial drugs ....................................................................................................................... 7 Antibacterial and antiprotozoal drugs ....................................................................................... 7 Systemic administration ........................................................................................................ 7 Topical administration ........................................................................................................... 9 Antifungal drugs ..................................................................................................................... -

The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men
ORIGINAL ARTICLE Endocrine Research The Effect of Gonadotropin Withdrawal and Stimulation with Human Chorionic Gonadotropin on Intratesticular Androstenedione and DHEA in Normal Men M. Y. Roth, S. T. Page, K. Lin, B. D. Anawalt, A. M. Matsumoto, B. Marck, W. J. Bremner, and J. K. Amory Downloaded from https://academic.oup.com/jcem/article/96/4/1175/2720870 by guest on 02 October 2021 Departments of Internal Medicine (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.) and Obstetrics and Gynecology (K.L.) and Center for Research in Reproduction and Contraception (M.Y.R., S.T.P., B.D.A., A.M.M., W.J.B., J.K.A.), University of Washington, Seattle, Washington 91895; and Geriatric Research (B.M.), Education and Clinical Center, Veterans Affairs Puget Sound Health Care System, Seattle, Washington 98105 Introduction: Concentrations of intratesticular (IT) testosterone (T) are known to be 100–200 times those of serum T; however, the IT concentrations of T’s precursors, their testicular to serum gra- dients, gonadotropin dependence, and response to stimulation with human chorionic gonado- tropin (hCG) have not been studied in detail. We hypothesized that serum and IT androstenedione (ADD) and IT dehydroepiandrosterone (DHEA) would be significantly suppressed by the adminis- tration of a GnRH antagonist and increased when stimulated by hCG, without a similar suppression of serum DHEA. Methods: We suppressed gonadotropins in 23 normal men with the GnRH antagonist acyline and randomly assigned them to one of four doses of hCG, 0, 15, 60, or 125 IU sc every other day for 10 d. -

Express Scripts 2020 National Preferred Formulary List
2020 Express Scripts National Preferred Formulary List The 2020 National Preferred Formulary drug list is shown below. The formulary is the list of drugs included in your prescription plan. Inclusion on the list does not guarantee coverage. The following list is not a complete list of over-the-counter [OTC] products and prescription medical supplies that are on the formulary. The only OTC products and prescription medical supplies that appear on the list are in contracted classes. PLEASE NOTE: Brand-name drugs may move to nonformulary status if a generic version becomes available during the year. Not all the drugs listed are covered by all prescription plans; check your benefit materials for the specific drugs covered and the copayments for your prescription plan. For specific questions about your coverage, please call the phone number printed on your member ID card. KEY For the member: FDA-approved generic medications meet strict standards and [INJ] – Injectable Drug contain the same active ingredients as their corresponding brand-name medications, [OTC] – Over-the-counter Product although they may have a different appearance. [SP] – Specialty Drug For the physician: Please prescribe preferred products and allow generic Brand-name drugs are listed in CAPITAL letters. Example: ABILIFY MAINTENA substitutions when medically appropriate. Generic drugs are listed in lower-case letters. Example: ibuprofen A adenosine [INJ] amabelz anthralin ADVAIR HFA amantadine APLISOL [INJ] abacavir [SP] ADVATE [INJ] [SP] AMBISOME [INJ] APOKYN [INJ] [SP] -

Orphan Drugs Used for Treatment in Pediatric Patients in the Slovak Republic
DOI 10.2478/v10219-012-0001-0 ACTA FACULTATIS PHARMACEUTICAE UNIVERSITATIS COMENIANAE Supplementum VI 2012 ORPHAN DRUGS USED FOR TREATMENT IN PEDIATRIC PATIENTS IN THE SLOVAK REPUBLIC 1Foltánová, T. – 2Konečný, M. – 3Hlavatá, A. –.4Štepánková, K. 5Cisárik, F. 1Comenius University in Bratislava, Faculty of Pharmacy, Department of Pharmacology and Toxicology 2Department of Clinical Genetics, St. Elizabeth Cancer Institute, Bratislava 32nd Department of Pediatrics, UniversityChildren'sHospital, Bratislava 4Slovak Cystic Fibrosis Association, Košice 5Department of Medical Genetics, Faculty Hospital, Žilina Due to the enormous success of scientific research in the field of paediatric medicine many once fatal children’s diseases can now be cured. Great progress has also been achieved in the rehabilitation of disabilities. However, there is still a big group of diseases defined as rare, treatment of which has been traditionally neglected by the drug companies mainly due to unprofitability. Since 2000 the treatment of rare diseases has been supported at the European level and in 2007 paediatric legislation was introduced. Both decisions together support treatment of rare diseases in children. In this paper, we shortly characterise the possibilities of rare diseases treatment in children in the Slovak republic and bring the list of orphan medicine products (OMPs) with defined dosing in paediatrics, which were launched in the Slovak market. We also bring a list of OMPs with defined dosing in children, which are not available in the national market. This incentive may help in further formation of the national plan for treating rare diseases as well as improvement in treatment options and availability of rare disease treatment in children in Slovakia.