Filovirus Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020 Taxonomic Update for Phylum Negarnaviricota (Riboviria: Orthornavirae), Including the Large Orders Bunyavirales and Mononegavirales
Archives of Virology https://doi.org/10.1007/s00705-020-04731-2 VIROLOGY DIVISION NEWS 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales Jens H. Kuhn1 · Scott Adkins2 · Daniela Alioto3 · Sergey V. Alkhovsky4 · Gaya K. Amarasinghe5 · Simon J. Anthony6,7 · Tatjana Avšič‑Županc8 · María A. Ayllón9,10 · Justin Bahl11 · Anne Balkema‑Buschmann12 · Matthew J. Ballinger13 · Tomáš Bartonička14 · Christopher Basler15 · Sina Bavari16 · Martin Beer17 · Dennis A. Bente18 · Éric Bergeron19 · Brian H. Bird20 · Carol Blair21 · Kim R. Blasdell22 · Steven B. Bradfute23 · Rachel Breyta24 · Thomas Briese25 · Paul A. Brown26 · Ursula J. Buchholz27 · Michael J. Buchmeier28 · Alexander Bukreyev18,29 · Felicity Burt30 · Nihal Buzkan31 · Charles H. Calisher32 · Mengji Cao33,34 · Inmaculada Casas35 · John Chamberlain36 · Kartik Chandran37 · Rémi N. Charrel38 · Biao Chen39 · Michela Chiumenti40 · Il‑Ryong Choi41 · J. Christopher S. Clegg42 · Ian Crozier43 · John V. da Graça44 · Elena Dal Bó45 · Alberto M. R. Dávila46 · Juan Carlos de la Torre47 · Xavier de Lamballerie38 · Rik L. de Swart48 · Patrick L. Di Bello49 · Nicholas Di Paola50 · Francesco Di Serio40 · Ralf G. Dietzgen51 · Michele Digiaro52 · Valerian V. Dolja53 · Olga Dolnik54 · Michael A. Drebot55 · Jan Felix Drexler56 · Ralf Dürrwald57 · Lucie Dufkova58 · William G. Dundon59 · W. Paul Duprex60 · John M. Dye50 · Andrew J. Easton61 · Hideki Ebihara62 · Toufc Elbeaino63 · Koray Ergünay64 · Jorlan Fernandes195 · Anthony R. Fooks65 · Pierre B. H. Formenty66 · Leonie F. Forth17 · Ron A. M. Fouchier48 · Juliana Freitas‑Astúa67 · Selma Gago‑Zachert68,69 · George Fú Gāo70 · María Laura García71 · Adolfo García‑Sastre72 · Aura R. Garrison50 · Aiah Gbakima73 · Tracey Goldstein74 · Jean‑Paul J. Gonzalez75,76 · Anthony Grifths77 · Martin H. Groschup12 · Stephan Günther78 · Alexandro Guterres195 · Roy A. -

MARBURG HEMORRHAGIC FEVER (Marburg HF)
MARBURG HEMORRHAGIC FEVER (Marburg HF) REPORTING INFORMATION • Class A: Report immediately via telephone the case or suspected case and/or a positive laboratory result to the local public health department where the patient resides. If patient residence is unknown, report immediately via telephone to the local public health department in which the reporting health care provider or laboratory is located. Local health departments should report immediately via telephone the case or suspected case and/or a positive laboratory result to the Ohio Department of Health (ODH). • Reporting Form(s) and/or Mechanism: o Immediately via telephone. o For local health departments, cases should also be entered into the Ohio Disease Reporting System (ODRS) within 24 hours of the initial telephone report to the ODH. • Key fields for ODRS reporting include: import status (whether the infection was travel-associated or Ohio-acquired), date of illness onset, and all the fields in the Epidemiology module. AGENT Marburg hemorrhagic fever is a rare, severe type of hemorrhagic fever which affects both humans and non-human primates. Caused by a genetically unique zoonotic RNA virus of the family Filoviridae, its recognition led to the creation of this virus family. The five species of Ebola virus are the only other known members of the family Filoviridae. Marburg virus was first recognized in 1967, when outbreaks of hemorrhagic fever occurred simultaneously in laboratories in Marburg and Frankfurt, Germany and in Belgrade, Yugoslavia (now Serbia). A total of 31 people became ill, including laboratory workers as well as several medical personnel and family members who had cared for them. -

Presentation
COMPLETE HEMORRHAGIC FEVER VIRUS INACTIVATION DURING LYSIS IN THE FILMARRAY BIOTHREAT-E ASSAY DEMONSTRATES THE BIOSAFETY OF THIS TEST. Olivier Ferraris (3), Françoise Gay-Andrieu (1), Marie Moroso (2), Fanny Jarjaval (3), Mark Miller (1), Christophe N. Peyrefitte (3) (1) bioMérieux, Marcy l’Etoile, France, (2) Fondation Mérieux, France (3) Unité de Virologie, Institut de recherche biomédicale des armées, Brétigny sur Orge, France Background : Viral hemorrhagic fevers (VHFs) are a group of illnesses caused by mainly five families of viruses namely Arenaviridae, Filoviridae , Bunyaviridae (Orthonairovirus genus ), Flaviviruses and Paramyxovirus (Henipavirus genus). The filovirus species known to cause disease in humans, Ebola virus (Zaire Ebolavirus), Sudan virus (Sudan Ebolavirus), Tai Forest virus (Tai Forest Ebolavirus), Bundibugyo virus (Bundibugyo Ebolavirus), and Marburg virus are restricted to Central Africa for 35 years, and spread to Guinea, Liberia, Sierra Leone in early 2014. Lassa fever is responsible for disease outbreaks across West Africa and in Southern Africa in 2008, with the identification of novel world arenavirus (Lujo virus). Henipavirus spread South Asia to Australia. CCHFv spread asia to south europa. They are transmitted from host reservoir by direct contacts or through vectors such as ticks bits. Working with VHF viruses, need a Biosafety Level 4 (BSL-4) laboratory, however during epidemics such observed with Ebola virus in 2014, the need to diagnose rapidly the patients raised the necessity to develop local laboratories These viruses represents a threat to healthcare workers and researches who manage infected diagnostic samples in laboratories. Aim : 1 Inactivation step An FilmArray Bio Thereat-E assay for detection of Hemorrhagic fever viruse Interfering substance HF virus + FA Lysis Buffer such as Ebola virus was developed to respond to Hemorrhagic fever virus 106 Ebola virus Whole blood + outbreak. -

Dengue Fever in Senegal 6 - 7 Ongoing Events Ebola Virus Disease in the Democratic Republic of the Congo Humanitarian Crisis in Cameroon
Overview Contents This Weekly Bulletin focuses on selected acute public health emergencies occurring in the WHO African Region. The WHO Health Emergencies Programme is currently monitoring 58 events in the region. This week’s edition covers key new and ongoing events, including: 2 Overview Hepatitis E in Central African Republic 3 - 5 New events Monkeypox in Central African Republic Dengue fever in Senegal 6 - 7 Ongoing events Ebola virus disease in the Democratic Republic of the Congo Humanitarian crisis in Cameroon. 8 Summary of major issues challenges and For each of these events, a brief description, followed by public health proposed actions measures implemented and an interpretation of the situation is provided. 9 All events currently A table is provided at the end of the bulletin with information on all new and being monitored ongoing public health events currently being monitored in the region, as well as events that have recently been closed. Major issues and challenges include: The Ebola virus disease (EVD) outbreak in the Democratic Republic of the Congo has reached a critical juncture, marked by a precarious security situation, persistence of pockets of community resistance/ mistrust and expanding geographical spread of the disease. During the reporting week, there was an incident involving a response team performing burial activity in Butembo. This came barely days following a widespread community strike (“ville morte”) in Beni and several towns, and an earlier armed attack in Beni. These incidents severely disrupted most outbreak control interventions. Meanwhile, EVD cases have been confirmed in new areas with worse insecurity and in close proximity to the border with Uganda. -

A Novel Ebola Virus VP40 Matrix Protein-Based Screening for Identification of Novel Candidate Medical Countermeasures
viruses Communication A Novel Ebola Virus VP40 Matrix Protein-Based Screening for Identification of Novel Candidate Medical Countermeasures Ryan P. Bennett 1,† , Courtney L. Finch 2,† , Elena N. Postnikova 2 , Ryan A. Stewart 1, Yingyun Cai 2 , Shuiqing Yu 2 , Janie Liang 2, Julie Dyall 2 , Jason D. Salter 1 , Harold C. Smith 1,* and Jens H. Kuhn 2,* 1 OyaGen, Inc., 77 Ridgeland Road, Rochester, NY 14623, USA; [email protected] (R.P.B.); [email protected] (R.A.S.); [email protected] (J.D.S.) 2 NIH/NIAID/DCR/Integrated Research Facility at Fort Detrick (IRF-Frederick), Frederick, MD 21702, USA; courtney.fi[email protected] (C.L.F.); [email protected] (E.N.P.); [email protected] (Y.C.); [email protected] (S.Y.); [email protected] (J.L.); [email protected] (J.D.) * Correspondence: [email protected] (H.C.S.); [email protected] (J.H.K.); Tel.: +1-585-697-4351 (H.C.S.); +1-301-631-7245 (J.H.K.) † These authors contributed equally to this work. Abstract: Filoviruses, such as Ebola virus and Marburg virus, are of significant human health concern. From 2013 to 2016, Ebola virus caused 11,323 fatalities in Western Africa. Since 2018, two Ebola virus disease outbreaks in the Democratic Republic of the Congo resulted in 2354 fatalities. Although there is progress in medical countermeasure (MCM) development (in particular, vaccines and antibody- based therapeutics), the need for efficacious small-molecule therapeutics remains unmet. Here we describe a novel high-throughput screening assay to identify inhibitors of Ebola virus VP40 matrix protein association with viral particle assembly sites on the interior of the host cell plasma membrane. -
![MARBURG VIRUS [African Hemorrhagic Fever, Green Or Vervet Monkey Disease]](https://docslib.b-cdn.net/cover/3334/marburg-virus-african-hemorrhagic-fever-green-or-vervet-monkey-disease-883334.webp)
MARBURG VIRUS [African Hemorrhagic Fever, Green Or Vervet Monkey Disease]
MARBURG VIRUS [African Hemorrhagic Fever, Green or Vervet Monkey Disease] SPECIES: Nonhuman primates, especially african green monkeys & macaques AGENT: Agent is classified as a Filovirus. It is an RNA virus, superficially resembling rhabdoviruses but has bizarre branching and filamentous or tubular forms shared with no other known virus group on EM. The only other member of this class of viruses is ebola virus. RESERVOIR AND INCIDENCE: An acute highly fatal disease first described in Marburg, Germany in 1967. Brought to Marburg in a shipment of infected African Green Monkeys from Uganda. 31 people were affected and 7 died in 1967. Exposure to tissue and blood from African Green monkeys (Cercopithecus aethiops) or secondary contact with infected humans led to the disease. No disease occurred in people who handled only intact animals or those who wore gloves and protective clothing when handling tissues. A second outbreak was reported in Africa in 1975 involving three people with no verified contact with monkeys. Third and fourth outbreaks in Kenya 1980 and 1987. Natural reservoir is unknown. Monkeys thought to be accidental hosts along with man. Antibodies have been found in African Green monkeys, baboons, and chimpanzees. 100% fatal in experimentally infected African Green Monkeys, Rhesus, squirrel monkeys, guinea pigs, and hamsters. TRANSMISSION: Direct contact with infected blood or tissues or close contact with infected patients. Virus has also been found in semen, saliva, and urine. DISEASE IN NONHUMAN PRIMATES: No clinical signs occur in green monkeys, but the disease is usually fatal after experimental infection of other primate species. Leukopenia and petechial hemorrhages throughout the body of experimentally infected monkeys, sometimes with GI hemorrhages. -

To Ebola Reston
WHO/HSE/EPR/2009.2 WHO experts consultation on Ebola Reston pathogenicity in humans Geneva, Switzerland 1 April 2009 EPIDEMIC AND PANDEMIC ALERT AND RESPONSE WHO experts consultation on Ebola Reston pathogenicity in humans Geneva, Switzerland 1 April 2009 © World Health Organization 2009 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distin- guished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. This publication contains the collective views of an international group of experts and does not necessarily represent the decisions or the policies of the World Health Organization. -

How Severe and Prevalent Are Ebola and Marburg Viruses?
Nyakarahuka et al. BMC Infectious Diseases (2016) 16:708 DOI 10.1186/s12879-016-2045-6 RESEARCHARTICLE Open Access How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence Luke Nyakarahuka1,2,5* , Clovice Kankya2, Randi Krontveit3, Benjamin Mayer4, Frank N. Mwiine2, Julius Lutwama5 and Eystein Skjerve1 Abstract Background: Ebola and Marburg virus diseases are said to occur at a low prevalence, but are very severe diseases with high lethalities. The fatality rates reported in different outbreaks ranged from 24–100%. In addition, sero-surveys conducted have shown different seropositivity for both Ebola and Marburg viruses. We aimed to use a meta-analysis approach to estimate the case fatality and seroprevalence rates of these filoviruses, providing vital information for epidemic response and preparedness in countries affected by these diseases. Methods: Published literature was retrieved through a search of databases. Articles were included if they reported number of deaths, cases, and seropositivity. We further cross-referenced with ministries of health, WHO and CDC databases. The effect size was proportion represented by case fatality rate (CFR) and seroprevalence. Analysis was done using the metaprop command in STATA. Results: The weighted average CFR of Ebola virus disease was estimated to be 65.0% [95% CI (54.0–76.0%), I2 = 97.98%] whereas that of Marburg virus disease was 53.8% (26.5–80.0%, I2 = 88.6%). The overall seroprevalence of Ebola virus was 8.0% (5.0%–11.0%, I2 = 98.7%), whereas that for Marburg virus was 1.2% (0.5–2.0%, I2 = 94.8%). -

A Look Into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins
viruses Review A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins Shanna S. Leventhal, Drew Wilson, Heinz Feldmann and David W. Hawman * Laboratory of Virology, Rocky Mountain Laboratories, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Hamilton, MT 59840, USA; [email protected] (S.S.L.); [email protected] (D.W.); [email protected] (H.F.) * Correspondence: [email protected]; Tel.: +1-406-802-6120 Abstract: In 2016, the Bunyavirales order was established by the International Committee on Taxon- omy of Viruses (ICTV) to incorporate the increasing number of related viruses across 13 viral families. While diverse, four of the families (Peribunyaviridae, Nairoviridae, Hantaviridae, and Phenuiviridae) contain known human pathogens and share a similar tri-segmented, negative-sense RNA genomic organization. In addition to the nucleoprotein and envelope glycoproteins encoded by the small and medium segments, respectively, many of the viruses in these families also encode for non-structural (NS) NSs and NSm proteins. The NSs of Phenuiviridae is the most extensively studied as a host interferon antagonist, functioning through a variety of mechanisms seen throughout the other three families. In addition, functions impacting cellular apoptosis, chromatin organization, and transcrip- tional activities, to name a few, are possessed by NSs across the families. Peribunyaviridae, Nairoviridae, and Phenuiviridae also encode an NSm, although less extensively studied than NSs, that has roles in antagonizing immune responses, promoting viral assembly and infectivity, and even maintenance of infection in host mosquito vectors. Overall, the similar and divergent roles of NS proteins of these Citation: Leventhal, S.S.; Wilson, D.; human pathogenic Bunyavirales are of particular interest in understanding disease progression, viral Feldmann, H.; Hawman, D.W. -
Weekly Bulletin on Outbreaks
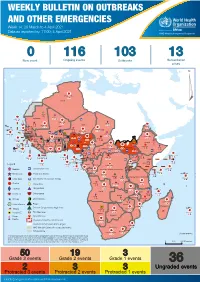
WEEKLY BULLETIN ON OUTBREAKS AND OTHER EMERGENCIES Week 14: 29 March to 4 April 2021 Data as reported by: 17:00; 4 April 2021 REGIONAL OFFICE FOR Africa WHO Health Emergencies Programme 0 116 103 13 New event Ongoing events Outbreaks Humanitarian crises 117 622 3 105 Algeria ¤ 36 13 110 0 5 420 164 Mauritania 7 2 10 501 392 110 0 7 0 Niger 17 927 449 Mali 3 334 10 567 0 6 0 2 079 4 4 595 165 Eritrea Cape Verde 38 520 1 037 Chad Senegal 4 918 185 59 0 Gambia 27 0 3 0 17 125 159 9 761 45 Guinea-Bissau 796 17 7 0 Burkina Faso 225 46 215 189 2 963 0 162 593 2 048 Guinea 12 817 150 12 38 397 1 3 662 66 1 1 23 12 Benin 30 0 Nigeria 1 873 71 0 Ethiopia 420 14 481 5 6 188 15 Sierra Leone Togo 3 473 296 53 920 779 52 14 Ghana 5 245 72 Côte d'Ivoire 10 098 108 14 484 479 63 0 40 0 Liberia 17 0 South Sudan Central African Republic 916 2 45 0 25 0 19 670 120 43 180 237 90 287 740 Cameroon 7 0 28 676 137 5 330 13 138 988 2 224 1 952 87 655 2 51 22 43 0 112 12 6 1 488 6 3 988 79 11 187 6 902 102 Equatorial Guinea Uganda 542 8 Sao Tome and Principe 32 11 2 042 85 41 016 335 Kenya Legend 7 100 90 Gabon Congo 18 504 301 Rwanda Humanitarian crisis 2 212 34 22 482 311 Measles 18 777 111 Democratic Republic of the Congo 9 681 135 Burundi 2 964 6 Monkeypox Ebola virus disease Seychelles 27 930 739 1 525 0 420 29 United Republic of Tanzania Lassa fever Skin disease of unknown etiology 189 0 4 084 20 509 21 Cholera Yellow fever 1 349 5 6 257 229 22 631 542 cVDPV2 Dengue fever 88 930 1 220 Comoros Angola Malawi COVID-19 Chikungunya 33 661 1 123 862 0 3 719 146 -

Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation
Ebola Virus Disease and Clinical Care Part I: History, Transmission, and Clinical Presentation This lecture is on Ebola virus disease (EVD) and clinical care. This is part one of a three-part lecture on this topic. Preparing Healthcare Workers to Work in Ebola Treatment Units (ETUs) in Africa This lecture will focus on EVD in the West African setting. Ebola Virus Disease and Clinical Care: The training and information you receive in this course will Part I: History, Transmission, and Clinical not cover the use of certain interventions such as intubation Presentation or dialysis which are not available in West African Ebola Treatment Units (ETUs). You will need supplemental training This presentation is current as of December 2014. This presentation contains materials from Centers for Disease Control and to care for patients appropriately in countries where advanced Prevention (CDC), Médecins Sans Frontières (MSF), and World Health Organization (WHO). care is available. U.S. Department of Health and Human Services U.S. Department of Health and Human Services Centers for Disease Control and Prevention Centers for Disease Control and Prevention version 12.03.2014 The learning objectives for this lecture are to: Learning Objectives ▶ Describe the routes of Ebola virus transmission Describe the routes of Ebola virus transmission Explain when and how patients are infectious ▶ Explain when and how patients are infectious Describe the clinical features of patients with Ebola ▶ Describe screening criteria for Ebola virus disease Describe the clinical features of patients with Ebola (EVD) used in West Africa Explain how to identify patients with suspected ▶ Describe screening criteria for EVD used in West Africa EVD who present to the ETU ▶ Explain how to identify patients with suspected EVD who present to the ETU This presentation contains materials from CDC, MSF, and WHO 2 A number of different viruses cause viral hemorrhagic fever. -

And Filoviruses Asit K
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Veterinary and Biomedical Science Veterinary and Biomedical Sciences, Department of 2016 Overview of Rhabdo- and Filoviruses Asit K. Pattnaik University of Nebraska-Lincoln, [email protected] Michael A. Whitt University of Tennessee Health Science Center, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/vetscipapers Part of the Biochemistry, Biophysics, and Structural Biology Commons, Cell and Developmental Biology Commons, Immunology and Infectious Disease Commons, Medical Sciences Commons, Veterinary Microbiology and Immunobiology Commons, and the Veterinary Pathology and Pathobiology Commons Pattnaik, Asit K. and Whitt, Michael A., "Overview of Rhabdo- and Filoviruses" (2016). Papers in Veterinary and Biomedical Science. 229. http://digitalcommons.unl.edu/vetscipapers/229 This Article is brought to you for free and open access by the Veterinary and Biomedical Sciences, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Veterinary and Biomedical Science by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in Biology and Pathogenesis of Rhabdo- and Filoviruses (2016), edited by Asit K Pattnaik and Michael A Whitt. Copyright © 2016 World Scientific Publishing Co Pte Ltd. Used by permission. digitalcommons.unl.edu CHAPTER 1 Overview of Rhabdo- and Filoviruses Asit K. Pattnaik1 and Michael A. Whitt2 1 School of Veterinary Medicine and Biomedical Sciences and Nebraska Center for Virology, University of Nebraska-Lincoln, Lincoln, Nebraska 68583 2 Department of Microbiology, Immunology, and Biochemistry, University of Tennessee Health Science Center, Memphis, Tennessee 38163 The authors contributed equally to this work. Emails: [email protected] ; [email protected] Summary Enveloped viruses with a negative-sense, single-stranded monopartite RNA genome have been classified into the orderMononegavirales .