Research Article Antibacterial Activity of Aqueous Extracts of Skimmia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Detailed Review on Morphotaxonomy And
Acta Scientific Microbiology (ISSN: 2581-3226) Review Article Volume 1 Issue 6 June 2018 Skimmia anquetilia N.P. Taylor and Airy Shaw A Detailed Review on Morphotaxonomy and Chemoprofiling of Saduf Nissar1, Neelofar Majid1, Aabid M Rather1*, Irshad A Nawchoo1 and GG Mohi-Ud-Din2 1Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, Department of Botany, University of Kashmir, Srinagar, India 2Department of Botany, Government Degree College for Women, Sopore, Baramullah, India *Corresponding Author: Aabid M Rather, Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, DepartmentReceived: April of Botany, 20, 2018; University Published: of Kashmir, May 28, Srinagar, 2018 India. Abstract - In recent times, medicinal plants have attracted huge attention due to their diverse range of biological and therapeutic proper Skimmia ties. Evidences have been accumulated since ages to demonstrate promising potential of medicinal plants used in various traditional, anquetilia complementary, and alternative systems with the ever-increasing interest of today’s population towards natural products, Rutaceae N.P. Taylor and Airy Shaw emerged out to be one of the most eye-catching plant bearing multiple medicinal properties. It is a perennial aromatic evergreen shrub belonging to family . Pharmacological studies have demonstrated significant action - of different extracts as antimicrobial, anti-inflammatory agents, among others, supporting some of its popular uses. An attempt has S. anquetilia been made in this review article to provide an up-to-date overview of the morphological parameters, taxonomic features, distribu tion pattern, traditional uses, as well as the phytochemistry and biological activities of . The present review provides insights for future research aiming for both ethnopharmacological validation of its popular use and its exploration as a new source ofKeywords herbal drugs: Skimmia and/or anquetilia; bioactive Rutaceaenatural products. -

Research Article Toxicological Evaluation of Essential Oils from Some Plants of Rutaceae Family
Hindawi Evidence-Based Complementary and Alternative Medicine Volume 2018, Article ID 4394687, 7 pages https://doi.org/10.1155/2018/4394687 Research Article Toxicological Evaluation of Essential Oils from Some Plants of Rutaceae Family Iram Liaqat ,1 Naila Riaz,2 Qurat-ul-Ain Saleem,2 Hafiz Muhammad Tahir,1 Muhammad Arshad,3 and Najma Arshad 2 1 Department of Zoology, Government College University, Lahore, Pakistan 2Department of Zoology, University of the Punjab, Quaid-e-Azam Campus, Lahore, Pakistan 3Department of Zoology, University of Education, Lahore, Pakistan Correspondence should be addressed to Najma Arshad; [email protected] Received 25 January 2018; Accepted 12 April 2018; Published 6 May 2018 Academic Editor: Nativ Dudai Copyright © 2018 Iram Liaqat et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Essential oils are produced as secondary metabolites by aromatic plants, predominantly belonging to families Apiaceae, Lamiaceae, Myrtaceae, and Rutaceae. Te family Rutaceae has great economic importance for its numerous edible fruits and essential oils. In the present study, essential oils of seven plants of family Rutaceae, Aegle marmelos, Murraya koenigii, Citrus reticulata Blanco, Zanthoxylum armatum, Skimmia laureola, Murraya paniculata,andBoenninghausenia albifora, were used for their toxicological assessment. Seven groups of selected essential oils-treated Wistar rats were established against control group (�=5) that received water for 14 days; animals were ofered feed and water ad libitum and treated with essential oils at 400 mg/kg body weight. Hematological studies revealed signifcant elevation in TEC in animals treated with essential oils of M. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -
Show Activity
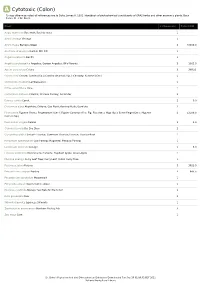
A Cytotoxic (Colon) *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Aegle marmelos Bael fruit; Bael de India 1 Ammi visnaga Visnaga 1 Ammi majus Bishop's Weed 2 40000.0 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 3 Angelica archangelica Angelica; Garden Angelica; Wild Parsnip 3 1902.0 Apium graveolens Celery 3 368.51 Carum carvi Carum; Comino (Sp.); Comino de prado (Sp.); Caraway; Kummel (Ger.) 1 Chenopodium album Lambsquarter 1 Citrus aurantiifolia Lime 1 Coriandrum sativum Cilantro; Chinese Parsley; Coriander 2 Daucus carota Carrot 2 6.0 Dictamnus albus Akgiritotu; Dittany; Gas Plant; Burning Bush; Gazelotu 2 Ficus carica Figueira (Port.); Feigenbaum (Ger.); Figuier Commun (Fr.); Fig; Fico (Ital.); Higo (Sp.); Echte Feige (Ger.); Higuera 2 12100.0 Comun (Sp.) Foeniculum vulgare Fennel 2 2.0 Glehnia littoralis Bei Sha Shen 2 Glycyrrhiza glabra Smooth Licorice; Commom Licorice; Licorice; Licorice-Root 1 Heracleum sphondylium Cow Parsnip; Hogweed; Meadow Parsnip 1 Levisticum officinale Lovage 1 6.0 Limonia acidissima Manzana De Elefante; Elephant Apple; Wood-Apple 1 Murraya koenigii Curry Leaf Tree; Curry Leaf; Indian Curry Tree 1 Pastinaca sativa Parsnip 3 3621.0 Petroselinum crispum Parsley 3 645.5 Peucedanum ostruthium Masterwort 1 Pimpinella anisum Sweet Cumin; Anise 1 Psoralea corylifolia Malaya Tea; Babchi; Black Dot 2 Ruta graveolens Rue 2 Skimmia japonica Japanese Skimmia 1 Zanthoxylum americanum Northern Prickly Ash 3 Zea mays Corn 1 Dr. Duke's Phytochemical and Ethnobotanical Databases Downloaded Tue Sep 28 01:08:32 EDT 2021 National Agricultural Library. -

First Steps Towards a Floral Structural Characterization of the Major Rosid Subclades
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2006 First steps towards a floral structural characterization of the major rosid subclades Endress, P K ; Matthews, M L Abstract: A survey of our own comparative studies on several larger clades of rosids and over 1400 original publications on rosid flowers shows that floral structural features support to various degrees the supraordinal relationships in rosids proposed by molecular phylogenetic studies. However, as many apparent relationships are not yet well resolved, the structural support also remains tentative. Some of the features that turned out to be of interest in the present study had not previously been considered in earlier supraordinal studies. The strongest floral structural support is for malvids (Brassicales, Malvales, Sapindales), which reflects the strong support of phylogenetic analyses. Somewhat less structurally supported are the COM (Celastrales, Oxalidales, Malpighiales) and the nitrogen-fixing (Cucurbitales, Fagales, Fabales, Rosales) clades of fabids, which are both also only weakly supported in phylogenetic analyses. The sister pairs, Cucurbitales plus Fagales, and Malvales plus Sapindales, are structurally only weakly supported, and for the entire fabids there is no clear support by the present floral structural data. However, an additional grouping, the COM clade plus malvids, shares some interesting features but does not appear as a clade in phylogenetic analyses. Thus it appears that the deepest split within eurosids- that between fabids and malvids - in molecular phylogenetic analyses (however weakly supported) is not matched by the present structural data. Features of ovules including thickness of integuments, thickness of nucellus, and degree of ovular curvature, appear to be especially interesting for higher level relationships and should be further explored. -

1980-04R.Pdf
COMING IN THE NEXT ISSUE Victoria Padilla is recognized as an expert on bromeliads. She will share her knowledge with readers in the OctoberlNovember issue when she writes about their history and development as popular house plants. In addition, look for George Taloumis' article on a charming Savannah townhouse garden and an article on new poinsettia varieties by another expert, Paul Ecke. Roger D. Way will write about new apple varieties and Mrs. Ralph Cannon will offer her G: hoices for hardy plants for damp soils. And last but not least, look for a staff article on money-saving ideas for the garden. We've canvassed over 100 gardeners for their best tips. All this and more in the next issue of American Horticulturist. Illustration by Vi rgini a Daley .- VOLUME 59 NUMBER 4 Judy Powell EDITO R Rebecca McClimans ART DIRECTOR Pam Geick PRODUCTION ASS ISTANT Steven H . Davis Jane Steffey ED ITO RI AL ASS ISTANTS H . Marc Cath ey Gi lbert S. Da ni els Donald Wyman H ORTICULTURAL CONSULTANTS Gil bert S. Daniels BOOK EDITOR Page 28 Page 24 May Lin Roscoe BUSINESS MA AGER Dorothy Sowerby EDUCATIONAL PROGRAMS FEATURES COORDINATOR Broad-leaved Evergreens 16 Judy Canady MEMBERSH IP/SUBSCRIPTI O N Text and Photograph y by Donald Wyman SERVICE Padua 18 Ci nd y Weakland Text and Photography by David W. Lee ASS IST ANT TO THE EDITOR John Si mm ons Bulbs That Last and Last 23 PRODUCTION C OORDINATIO N Isabel Zucker Chro magraphics In c. Plant Propagation-The Future is Here 24 COLOR SEPARATI ONS Chiko Haramaki and Charles Heuser C. -

The Himalayan Species of Skimmia Author(S): J
The Himalayan Species of Skimmia Author(s): J. S. Gamble Source: Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew), Vol. 1917, No. 9/10 (1917), pp. 301-303 Published by: Springer on behalf of Royal Botanic Gardens, Kew Stable URL: http://www.jstor.org/stable/4113574 Accessed: 27-06-2016 06:50 UTC Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://about.jstor.org/terms JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Royal Botanic Gardens, Kew, Springer are collaborating with JSTOR to digitize, preserve and extend access to Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew) This content downloaded from 131.247.112.3 on Mon, 27 Jun 2016 06:50:39 UTC All use subject to http://about.jstor.org/terms 301 XXX.-THE HIMALAYAN SPECIES OF SKIMMIA. J. S. GAMBLE. For some years I have been under the impression that the plant described in the Flora of British India I. p. 499 (1875) as Skimmia Laureola contained more than one species, because, in addition to the well-known undershrub of the Western Himalaya, barely 2-3 ft. high with pale yellow flowers and red berries, I found in the Eastern Himalaya one which grew into a small tree and had nearly white flowers and black berries, while at high levels also in the Eastern Himalaya there seemed to be a third, a quite low trailing shrub also with whitish flowers and (so far as I know) greenish rather dry berries. -

3250. Citrus Pests
CALIFORNIA DEPARTMENT OF FOOD AND AGRICULTURE 301.1 PLANT QUARANTINE MANUAL 09-17-12 3250. CITRUS PESTS State Exterior Quarantine A quarantine is established against the following pests, their indicating the fruit was treated in accordance with hosts and possible carriers. methods approved by the department (Also see APPENDIX B). A. Pests. Any species of fruit flies of the family Tephritidae known to attack citrus; citrus canker, Xanthomonas b. Shipments of Texas citrus fruit, except lemons and axonopodis pv. citri; and any other injurious insect or other sour limes, must be accompanied by a Federal animal or plant disease pest of citrus which does not occur, or Master Permit issued under provisions of the is not generally established in California. United States Mexican Fruit Fly Quarantine and by an agent of the United States Department of B. Area Under Quarantine. All states, districts, and Agriculture (Also see APPENDIX D). territories of the United States, except the State of Arizona. c. Surface Pests. Treatment for surface pests (scale, C. Articles and Commodities Covered. insects, mites, etc.) is not required as a condition 1. From the area under quarantine, except the State of of entry for all citrus fruit, including lemons and Florida: sour limes, from Florida and Texas destined to California when the fruit has been cleaned by ; a. All species and varieties of citrus fruits washing and scrubbing with brushes in a b. All plants and propagative parts, except seed, commercial packing house in preparation for belonging to, or hybrids of, the genera Citrus (true interstate shipment. If the fruit has not been so citrus), Fortunella (kumquats), Poncirus (trifoliate cleaned, prepared and handled, then the fruit shall oranges), Aeglopsis (dwarf powder-flask fruit), and be treated to assure the fruit is free of surface Afraegle (African powder-flask fruit). -

BMC Evolutionary Biology Biomed Central
BMC Evolutionary Biology BioMed Central Research article Open Access Mitochondrial matR sequences help to resolve deep phylogenetic relationships in rosids Xin-Yu Zhu1,2, Mark W Chase3, Yin-Long Qiu4, Hong-Zhi Kong1, David L Dilcher5, Jian-Hua Li6 and Zhi-Duan Chen*1 Address: 1State Key Laboratory of Systematic and Evolutionary Botany, Institute of Botany, the Chinese Academy of Sciences, Beijing 100093, China, 2Graduate University of the Chinese Academy of Sciences, Beijing 100039, China, 3Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, UK, 4Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, MI 48108-1048, USA, 5Florida Museum of Natural History, University of Florida, Gainesville, FL 32611-7800, USA and 6Arnold Arboretum of Harvard University, 125 Arborway, Jamaica Plain, MA 02130, USA Email: Xin-Yu Zhu - [email protected]; Mark W Chase - [email protected]; Yin-Long Qiu - [email protected]; Hong- Zhi Kong - [email protected]; David L Dilcher - [email protected]; Jian-Hua Li - [email protected]; Zhi- Duan Chen* - [email protected] * Corresponding author Published: 10 November 2007 Received: 19 June 2007 Accepted: 10 November 2007 BMC Evolutionary Biology 2007, 7:217 doi:10.1186/1471-2148-7-217 This article is available from: http://www.biomedcentral.com/1471-2148/7/217 © 2007 Zhu et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

Phylogenetic Analysis Reveals a Scattered Distribution of Autumn Colours
Annals of Botany 103: 703–713, 2009 doi:10.1093/aob/mcn259, available online at www.aob.oxfordjournals.org Phylogenetic analysis reveals a scattered distribution of autumn colours Marco Archetti* Department of Zoology, Oxford University, South Parks Road, Oxford OX1 3PS, UK Received: 1 September 2008 Returned for revision: 24 October 2008 Accepted: 25 November 2008 Published electronically: 6 January 2009 † Background and Aims Leaf colour in autumn is rarely considered informative for taxonomy, but there is now growing interest in the evolution of autumn colours and different hypotheses are debated. Research efforts are hindered by the lack of basic information: the phylogenetic distribution of autumn colours. It is not known when and how autumn colours evolved. † Methods Data are reported on the autumn colours of 2368 tree species belonging to 400 genera of the temperate regions of the world, and an analysis is made of their phylogenetic relationships in order to reconstruct the evol- utionary origin of red and yellow in autumn leaves. † Key Results Red autumn colours are present in at least 290 species (70 genera), and evolved independently at least 25 times. Yellow is present independently from red in at least 378 species (97 genera) and evolved at least 28 times. † Conclusions The phylogenetic reconstruction suggests that autumn colours have been acquired and lost many times during evolution. This scattered distribution could be explained by hypotheses involving some kind of coevolutionary interaction or by hypotheses that rely on the need for photoprotection. Key words: Autumn colour, leaf colour, comparative analysis, coevolution, photoprotection, phylogenetic analysis. INTRODUCTION all year round and become visible during leaf senescence because of the degradation of chlorophyll (Biswal, 1995), antho- Autumn colours cyanins are actively produced in autumn (Sanger, 1971; Lee, The colour change of leaves in autumn is a spectacular 2002; Lee and Gould, 2002). -

2016 Living Collections Inventory N
Box 561, West Tisbury, MA 02575 508-693-9426 www.pollyhillarboretum.org Liriodendron tulipifera – Tulip-tree (PHA acc. #58-003-01A) 2016 LIVING COLLECTIONS INVENTORY N West Woodland S West Woodland N “Yellow Corner” WFSW WFW N1/2 WFW S1/2 West Field PICNIC GROVE Littlefield 1 2 3 4 5 6 Nursery WFSE HP5 EW SW NW HP4 HP3 VCW LMB ARB-W HP1 SE WEST ARBST HP2 VCN EN NE ONEG VEG-FLD-W ES ARB- VCE CTR Far PINE GROVE EE Barn CEDAR –G C TA BOWER NURS-FLD-W NORTH FIELD W ARB-E VEG-FLD-E Cow Barn HOMESTEAD S LH BARN YARD North Field NURS-FLD-E P NORTH FIELD E CODE LOCATION COMMENTS ALLEE Cornus Kousa Allee ARB-CTR Arboretum Center ARB-E Arboretum East ARB-N Arboretum North ARB-S Arboretum South ARB-W Arboretum West ARBSTONEG Arboretum - Stone Garden BARNYARD Barnyard BORDER Border BOWER Bower C Container area not open to the public CEDAR-G Cedar Garden CR1 Conifer Row 1 CR2 Conifer Row 2 CR3 Conifer Row 3 CR4 Conifer Row 4 CR5 Conifer Row 5 CR6 Conifer Row 6 EE Entrance East EL-FLD-E Ell Field East EL-FLD-N Ell Field North EL-FLD-S Ell Field South EL-FLD-W Ell Field West EN Entrance North ES Entrance South EW Entrance West HB Homestead Border HOMESTEADE Homestead East HOMESTEADS Homestead South HP1 Holly Park Bed 1 Hilly's Garden HP2 Holly Park Bed 2 Hilly's Garden HP3 Holly Park Bed 3 Hilly's Garden HP4 Holly Park Bed 4 Hilly's Garden CODE LOCATION COMMENTS HP5 Holly Park Bed 5 / Hilly's Garden LH Littlefield House area not open to the public LMB EAST Littlefield Maintenance Building East LMB WEST Littlefield Maintenance Building West LNNE1 Littlefield -

3250. Citrus Pests
CALIFORNIA DEPARTMENT OF FOOD AND AGRICULTURE 301.1 PLANT QUARANTINE MANUAL 11-30-10 3250. CITRUS PESTS State Exterior Quarantine Department of Agriculture and Consumer Services indicating the fruit was treated in accordance with A quarantine is established against the following pests, their hosts and possible carriers. methods approved by the department (Also see APPENDIX B). A. Pests. Any species of fruit flies of the family Tephritidae known to attack citrus; citrus canker, Xanthomonas b. Shipments of Texas citrus fruit, except lemons and axonopodis pv. citri; and any other injurious insect or other sour limes, must be accompanied by a Federal animal or plant disease pest of citrus which does not occur, or Master Permit issued under provisions of the is not generally established in California. United States Mexican Fruit Fly Quarantine and by an agent of the United States Department of B. Area Under Quarantine. All states, districts, and Agriculture (Also see APPENDIX D). territories of the United States, except the State of Arizona. c. Surface Pests. Treatment for surface pests (scale, C. Articles and Commodities Covered. insects, mites, etc.) is not required as a condition 1. From the area under quarantine, except the State of of entry for all citrus fruit, including lemons and Florida: sour limes, from Florida and Texas destined to California when the fruit has been cleaned by a. All species and varieties of citrus fruits; washing and scrubbing with brushes in a b. All plants and propagative parts, except seed, commercial packing house in preparation for belonging to, or hybrids of, the genera Citrus (true interstate shipment.