L43 Official Journal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UCHWAŁA Nr 28/2018 ZARZĄDU WOJEWÓDZTWA WIELKOPOLSKIEGO Z Dnia 7 Grudnia 2018 Roku
UCHWAŁA Nr 28/2018 ZARZĄDU WOJEWÓDZTWA WIELKOPOLSKIEGO z dnia 7 grudnia 2018 roku w sprawie zaopiniowania projektu Programu Ochrony Środowiska dla Powiatu Szamotulskiego na lata 2018-2021 z perspektywą do 2025 roku Na podstawie art. 17 ust. 2 pkt. 2 ustawy z dnia 27 kwietnia 2001 r. Prawo ochrony środowiska (Dz. U. z 2017 r. poz. 519 ze zm.) Zarząd Województwa Wielkopolskiego uchwala, co następuje: § 1. Opiniuje się pozytywnie projekt Programu Ochrony Środowiska dla Powiatu Szamotulskiego na lata 2018-2021 z perspektywą do 2025 roku. § 2. Postanawia się przekazać niniejszą uchwałę Zarządowi Powiatu Szamotulskiego w celu przeprowadzenia dalszego postępowania. § 3. Wykonanie uchwały powierza się Dyrektorowi Departamentu Środowiska Urzędu Marszałkowskiego Województwa Wielkopolskiego. § 4. Uchwała wchodzi w życie z dniem podjęcia. z up. Marszałka Województwa Wojciech Jankowiak Wicemarszałek UZASADNIENIE do uchwały Nr 28/2018 Zarządu Województwa Wielkopolskiego z dnia 7 grudnia 2018 roku Przepis art. 17 ust. 1 ustawy z dnia 27 kwietnia 2001 r. Prawo ochrony środowiska stanowi, że w celu realizacji polityki ochrony środowiska opracowuje się programy ochrony środowiska. Programy są opracowywane na szczeblu wojewódzkim, powiatowym i gminnym oraz podlegają zaopiniowaniu przez odpowiednie organy administracji. Wykonując kompetencje art. 17 ust. 2 pkt. 2 ustawy Prawo ochrony środowiska Starosta Szamotulski, reprezentowany przez pełnomocnika, zwrócił się z wnioskiem o zaopiniowanie Programu Ochrony Środowiska dla Powiatu Szamotulskiego na lata 2018-2021 z perspektywą do 2025 roku. Zarząd Województwa Wielkopolskiego po przeanalizowaniu przekazanej dokumentacji i obowiązującego stanu prawnego zaopiniował pozytywnie projekt Programu Ochrony Środowiska dla Powiatu Szamotulskiego na lata 2018-2021 z perspektywą do 2025 roku i postanowił przekazać niniejszą uchwałę Zarządowi Powiatu Szamotulskiego w celu przeprowadzenia dalszego postępowania. -

Zarzadzenie 34/2021
DZIENNIK URZĘDOWY WOJEWÓDZTWA WARMIŃSKO-MAZURSKIEGO Olsztyn, dnia 26 kwietnia 2021 r. Poz. 1538 ZARZĄDZENIE NR 34/2021 WÓJTA GMINY GRUNWALD z dnia 30 marca 2021 r. w sprawie przyjęcia sprawozdania rocznego z wykonania budżetu Gminy Grunwald za 2020 rok Na podstawie art. 267 i art. 269 ustawy z dnia 27 sierpnia 2009 roku o finansach publicznych (t.j. Dz. U. z 2021 r. poz. 305) Wójt Gminy zarządza, co następuje: § 1. Przyjmuję sprawozdanie roczne z wykonania budżetu Gminy Grunwald za 2020 r. jak w załącznikach od Nr 1 do Nr 16. § 2. Sprawozdanie, o którym mowa w § 1 Wójt Gminy przekaże do dnia 31 marca 2021 r. Radzie Gminy i Regionalnej Izbie Obrachunkowej w Olsztynie. § 3. Zarządzenie wchodzi w życie z dniem podjęcia i podlega ogłoszeniu w Dzienniku Urzędowym Województwa Warmińsko-Mazurskiego. Wójt Adam Szczepkowski Dziennik Urzędowy Województwa Warmińsko-Mazurskiego – 2 – Poz. 1538 Załącznik Nr 1 do Zarządzenia Nr 34/2021 Wójta Gminy Grunwald z dnia 30 marca 2021 r. WYKONANIE PLANU DOCHODÓW B u d ż e t W y k o n a n i e Dz. Rozdz Paragr. Nazwa po zmianach kwota strukt 2:1 -1- -2- % % 010 ROLNICTWO I ŁOWIECTWO 875 458,14 870 859,96 2,4 99,5 Infrastruktura wodociagowa i 01010 8 390,00 8 364,00 1,0 99,7 sanitacyjna wsi 0920D Pozostałe odsetki 26,00 0,00 0,0 0,0 Dotacje celowe w ramach programów finansowych z udziałem środków europejskich oraz środków, o których 6257D mowa w art. 5 ust. 3 pkt 5 lit. a i b 8 364,00 8 364,00 100,0 100,0 ustawy, lub płatności w ramach budżetu środków europejskich, realizowanych przez jednostki samorządu terytorialnego -

Gmina Rakoniewice
TONSMEIER ZACHÓD Sp. z o.o. Tel. 61 442 49 73, 65 512 75 31 ODBIÓR ODPADÓW MIEJSCOWOŚĆ/ULICA WIELKOGABARYTOWYCH ( GMINA RAKONIEWICE ZBIÓRKA 2 X W ROKU SPRZED POSESJI) Nieruchomości zamieszkałe - ZABUDOWA JEDNORODZINNA - częstotliwość co 2 tygodnie O ROK 2017 III IV V VI VII VIII IX X XI XII Głodno, Gola, Stodolsko, Tarnowa 13, 27 10, 24 8, 22 5, 19 3, 17 ,31 14, 28 11, 25 9, 23 6, 20 4, 18 3.04.2017 2.10.2017 D Gnin, Rataje, Ruchocice 14, 28 11, 25 9, 23 6, 20 4, 18 1, 16, 29 12, 26 10, 24 7, 21 5, 19 4.04.2017 3.10.2017 P Komorówko, Jabłonna 1,15, 29 12, 26 10 ,24 7, 21 5, 19 2, 17, 30 13, 27 11, 25 8, 22 6, 20 5.04.2017 4.10.2017 A os.Parkowe, Wygoda, Dadaczyńskiego, D Sikorskiego, Tetmajera, Mickiewicza, Sienkiewicza, Rożka, Kocha, Jana Kazimierza, Y Słowackiego, Reymonta, Rzemieślnicza, 2, 16, 30 13, 27 11, 25 8, 22 6, 20 3, 18, 31 14 ,28 12, 26 9, 23 7, 21 Jasińskiego, Iwaszkiewicza, Kochanowskiego, Czereśniowa, Brzoskwiniowa, Nowotomyska, N Wolsztyńska, Starotomyska, Garbary, Szkolna, I Piekary, Różana, Gregora, Kościelna, Kamińskiego 6.04.2017 5.10.2017 E Rostarzewo 3, 17, 31 14, 28 12, 26 9, 23 7, 21 4, 19 1, 15, 29 13, 27 10, 24 8, 22 7.04.2017 6.10.2017 6, 20 3, 18, 29 15, 29 12, 26 10, 24 7, 21 4, 18 2, 16, 30 13, 27 11, 23 S Adolfowo, Cegielsko, Łąkie, Łąkie Nowe 3.04.2017 2.10.2017 E Aleksandrowo Leśniczówka, Błońsko, Krzewina 7, 21 4, 19 2, 16, 30 13, 27 11, 25 8, 22 5, 19 3, 17, 31 14, 28 12, 27 Leśniczówka, Kuźnica Zbąska, Wola Jabłońska 4.04.2017 3.10.2017 G Blinek, Elżbieciny, Wioska 8, 22 5, 20 4, 17, 31 14 ,28 12, 26 9, 23 6, 20 4, 18 2, 15, 29 13, 28 5.04.2017 4.10.2017 R E Rakoniewice Leśniczówka, Rakoniewice Wieś, Wielichowska, Grodziska, Wiatrakowa, Plac G Powst. -

Dzielnicowi Kpp Szamotuły
KPP SZAMOTUŁY https://szamotuly.policja.gov.pl/w26/twoj-dzielnicowy/dzielnicowi-kpp-szamotu/127786,Dzielnicowi-KPP-Szamotuly.html 2021-09-30, 16:26 DZIELNICOWI KPP SZAMOTUŁY Kierownik Rewiru Dzielnicowych Komendy Powiatowej Policji w Szamotułach asp. sztab. Mariusz Sołtysiak Rejon nr 1 dzielnicowy st. sierż. Kamil Krych tel. 47 77 32 227 lub 786 936 496 e-mail: [email protected] W skład rejonu wchodzą ulice: Owsiana, Gruszowa,Pszenna, Kopernika, Jęczmienna, Strzeleckiego, Graniczna,Łukasiewicza, Konstantego Scholl, M.C. Skłodowskiej, Długa,Święcickiego, Szczuczyńska, Śniadeckich, Wierzbowa, Wiśniowa,Topolowa, Zakole, Zielona, Brzozowa, Akacjowa, Bukowa, Klonowa,Kasztanowa, Jaworowa, Dębowa, Leśna, Gajowa, Borowa,Moraczewskiego, Halszki, Calliera, Powstańców Wlkp.,Fryderyka Chopina, Boczna, Wiejska, Rzemieślnicza, Miła, Dobra,Wiosny Ludów, Kalinowa, Powstania Styczniowego – Różana,Jaśminowa, Ogrodowa, Polna, Sezamkowa, Zamkowa, Podgórna,Skryta, Cicha, Cienista, Jabłoniowa, Czereśniowa, Morelowa. Rejon nr 2 dzielnicowy mł. asp. Hubert Dominiak tel. 47 77 32 227 lub 786 936 492 e-mail: [email protected] W skład rejonu wchodzą ulice: Szamotułyulice - Aleja 1 Maja, Braci Czeskich, Cmentarna, Targowa, PlacSienkiewicza, Wroniecka, Kapłańska, Kościelna, Wodna, Garncarska,Piekarska, Wąska, Rynek, Piotra Skargi, Ratuszowa, Sukiennicza,Rynkowa, Średnia, Krótka, Szkolna, Krzywa, Poznańska,Dworcowa (1-11). Rejon nr 3 dzielnicowy mł. asp. Dawid Serwata tel. 47 77 32 227 lub 786 936 493 e-mail: [email protected] -

Poolęderskie Budownictwo Mieszkalne Na Obszarze Równiny Nowotomyskiej*
STUDIA LEDNICKIE IV Poznań — Lednica 1996 ANTONI PELCZYK ' Muzeum Pierwszych Piastów na Lednicy POOLĘDERSKIE BUDOWNICTWO MIESZKALNE NA OBSZARZE RÓWNINY NOWOTOMYSKIEJ* Drei Häuser hat mir Gott gegeben; In diesem will ich ewig leben. In jenem will ich ruhen fein. Im dritten will ich ewig sein} W krajobrazie osadniczym Wielkopolski szczególną odrębnością środowiska przy rodniczego oraz budownictwa wiejskiego charakteryzuje się Równina Nowotomyska potocznie zwana niecką nowotomyską. Rejon ten zamknięty mniej więcej linią: Pniewy-Lwówek-Pszczew-Trzciel-Zbą- szyń-Wolsztyn-Rakoniewice, od początku XVIII wieku stał się terenem intensywnego osadnictwa oięderskiego, którego ślady uchwytne są jeszcze dzisiaj (zob. ryc. 1). Obszar ten o przewadze gleb wytworzonych na glinach zwałowych i piaskach, w większości podzielony na małe zagłębienia oddzielone pasami piaszczystych usypisk wydmowych, poprzecinany licznymi rowami i strumykami, wymagający prac melioracyjnych i po kryty puszczą — przez długi czas nie przedstawiał dla osadnictwa rolniczego większej wartości (zob. ryc. 2 i 3). Stąd też, jedynie na skraju wydzielonego obszaru znajdowały się tereny od dawna zasiedlone, niewielkie zaś enklawy tworzyły wydarte puszczy tereny wokół Tomyśla (obecnie Stary Tomyśl), Wytomyśla, Grudny, Boruji, Łomnicy, Kąkolewa, Kuślina, Bolewic, Chmielinka, Jabłonnej2. Stan ten uległ diametralnej zmia nie dopiero w dobie wielkiego ruchu kolonizacyjnego trwającego w Wielkopolsce z niedługimi przerwami przez wiek XVII i XVIII aż do rozbiorów Polski. W tym ♦Niniejszy artykuł stanowią fragmenty przygotowanego do druku opracowania Budownictwo olęderskie na obszarze Równiny Nowolomyskiej, które ukaże się (w:) Budownictwo ludowe w Polsce. Seria mono graficzna. Muzeum Budownictwa Ludowego, Sanok. 1 Inskrypcja na belce XVIII-wiecznej chałupy z Marianowa (Mariendorf, Netzekreis) (wg P. Groth 1939, s. 43). 2Szczegółowsze informacje podają: J. Burszta 1958, s. -

(EU) 2020/281 of 27 February 2020 Amending the Annex To
28.2.2020 EN Offi cial Jour nal of the European Union L 59/13 COMMISSION IMPLEMENTING DECISION (EU) 2020/281 of 27 February 2020 amending the Annex to Implementing Decision (EU) 2020/47 on protective measures in relation to highly pathogenic avian influenza of subtype H5N8 in certain Member States (notified under document C(2020) 1248) (Text with EEA relevance) THE EUROPEAN COMMISSION, Having regard to the Treaty on the Functioning of the European Union, Having regard to Council Directive 89/662/EEC of 11 December 1989 concerning veterinary checks in intra-Community trade with a view to the completion of the internal market (1), and in particular Article 9(4) thereof, Having regard to Council Directive 90/425/EEC of 26 June 1990 concerning veterinary checks applicable in intra-Union trade in certain live animals and products with a view to the completion of the internal market (2), and in particular Article 10(4) thereof, Whereas: (1) Commission Implementing Decision (EU) 2020/47 (3) was adopted following outbreaks of highly pathogenic avian influenza of subtype H5N8 in holdings where poultry are kept in certain Member States, and the establishment of protection and surveillance zones by those Member States in accordance with Council Directive 2005/94/EC (4). (2) Implementing Decision (EU) 2020/47 provides that the protection and surveillance zones established by the Member States listed in the Annex to that Implementing Decision, in accordance with Directive 2005/94/EC, are to comprise at least the areas listed as protection and surveillance zones in that Annex. (3) The Annex to Implementing Decision 2020/47 was recently amended by Commission Implementing Decision (EU) 2020/240 (5), following outbreaks of highly pathogenic avian influenza of subtype H5N8 in poultry in Bulgaria and Czechia that needed to be reflected in that Annex. -

Uchwała Nr Xxxix/348/2010 Rady Miejskiej W Rakoniewicach
UCHWAŁA NR XXXIX/348/2010 RADY MIEJSKIEJ W RAKONIEWICACH z dnia 10 sierpnia 2010 r. w sprawie: stałych obwodów głosowania Na podstawie art. 30 ust. 2 ustawy z dnia 16 lipca 1998 r. - Ordynacja wyborcza do rad gmin, rad powiatów i sejmików województw (Dz. U. z 2003 r. Nr 159, poz. 1547 z późn. zm.) oraz art. 40 ust. 1 ustawy z dnia 8 marca 1990 r. o samorządzie gminnym (Dz. U. z 2001 r. Nr 142, poz. 1591, z późn. zm.) Rada Miejska w Rakoniewicach na wniosek Burmistrza Rakoniewic uchwala co następuje: § 1. Dokonuje się zmiany w stałych obwodach głosowania. § 2. Podział na stałe obwody głosowania, ich granice i numery oraz siedziby obwodowych komisji wyborczych określa załącznik do niniejszej uchwały. § 3. Wykonanie uchwały powierza się Burmistrzowi Rakoniewic. § 4. Traci moc uchwała Nr XXII/134/2000 Rady Miejskiej Gminy Rakoniewice z dnia 22 sierpnia 2000r. w sprawie zmiany granic obwodów głosowania. § 5. Uchwała podlega niezwłocznemu przekazaniu Wojewodzie Wielkopolskiemu i Komisarzowi Wyborczemu w Lesznie. § 6. Uchwała podlega ogłoszeniu w Dzienniku Urzędowym Województwa Wielkopolskiego i podaniu do publicznej wiadomości w sposób zwyczajowo przyjęty w gminie. § 7. Uchwała wchodzi w życie po upływie 14 dni od dnia ogłoszenia w Dzienniku Urzędowym Województwa Wielkopolskiego. Id: PMDQJ-BJDDY-AGIJB-UVHXM-QFNAQ. Podpisany Strona 1 Załącznik Nr 1 do Uchwały Nr XXXIX/348/2010 Rady Miejskiej w Rakoniewicach z dnia 10 sierpnia 2010 r. Stałe obwody głosowania, ich granice i numery na terenie miasta i gminy Rakoniewice Numer obwodu Granice obwodu głosowania /miejscowości/ głosowania Rakoniewice miasto, ulice: Cmentarna, Dębowa, Dworcowa, Kolejowa, Kostki, Krystyny, Kusocińskiego, Malinowa, Ogrodowa, Osiedle Drzymały, Piaskowa, Piekary, Plac Powstańców Wielkopolskich, Pocztowa, Polna, Przemysłowa, Różana, 1 Słoneczna, Sportowa, Stadion, Starowolsztyńska, Strzelecka, Wiatrakowa, Wielichowska, Wolsztyńska, Zamkowa, Rakoniewice Osiedle Parkowe. -

OBWIESZCZENIE BURMISTRZA MIASTA I GMINY SZAMOTUŁY Z Dnia 18 Września 2018 R
OBWIESZCZENIE BURMISTRZA MIASTA I GMINY SZAMOTUŁY z dnia 18 września 2018 r. Na podstawie art. 16 § 1 ustawy z dnia 5 stycznia 2011 r. – Kodeks wyborczy (Dz. U. z 2018 r. poz. 754, 1000 i 1349) Burmistrz Miasta i Gminy Szamotuły podaje do wiadomości wyborców informację o numerach oraz granicach obwodów głosowania, wyznaczonych siedzibach obwodowych komisji wyborczych oraz możliwości głosowania korespondencyjnego i przez pełnomocnika w wyborach do rad gmin, rad powiatów i sejmików województw oraz w wyborach wójtów, burmistrzów i prezydentów miast zarządzonych na dzień 21 października 2018 r.: A: Stałe obwody głosowania Numer obwodu Nazwa i siedziba obwodowej komisji wyborczej * Granice obwodu głosowania (obszar obwodu głosowania) Obwodowa Komisja Wyborcza nr 1 w Kąsinowie Baborowo, Baborówko, Gąsawy, Nowy Folwark, Kąsinowo, Kępa, Myszkowo, Obwód głosowania Kąsinowo 54, 64-500 Szamotuły Piaskowo nr 1 (ŚWIETLICA WIEJSKA W KĄSINOWIE) Obwodowa Komisja Wyborcza nr 2 w Pamiątkowie Lulinek, Pamiątkowo, Rudnik, Przecławek, Przecław, Witoldzin, Żalewo Obwód głosowania ul. Szkolna 13, 64-514 Pamiątkowo nr 2 (SZKOŁA PODSTAWOWA W PAMIĄTKOWIE) Obwodowa Komisja Wyborcza nr 3 w Otorowie Koźle, Krzeszkowice, Lipnica, Czyściec, Bociany, Lipnickie Huby, Otorowo, Obwód głosowania ul. Łąkowa 2A, 64-551 Otorowo Kamionka, Mielno, Poświętne, Wincentowo nr 3 (ŚWIETLICA WIEJSKA W OTOROWIE) Obwodowa Komisja Wyborcza nr 4 w Gałowie Brodziszewo, Emilianowo, Gałowo, Gałowo-Majątek, Jastrowo, Jastrowo-Majątek, Obwód głosowania Gałowo, ul. Wierzbowa 10, 64-500 Szamotuły Ostrolesie, Przyborowo, Przyborówko nr 4 (PRH "GAŁOPOL" Sp. z o.o.) Obwodowa Komisja Wyborcza nr 5 w Szamotułach Mutowo, Piotrkówko, Szczuczyn, Śmiłowo, Ludwikowo, Grabowiec, Twardowo Obwód głosowania ul. Szczuczyńska 3, 64-500 Szamotuły nr 5 (ZESPÓŁ SZKÓŁ NR 2 W SZAMOTUŁACH) Szamotuły - ulice: Cmentarna, Hetmańska, Jastrowska, Jaśminowa, Jesienna, Obwodowa Komisja Wyborcza nr 6 w Szamotułach Kalinowa, Kosynierów, Letnia, 1 Maja, Ogrodowa, Ostrorogska, Polna, Powstania Obwód głosowania ul. -
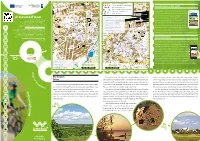
Web Portal Z P R R P Wojennych Ie I Z BRZESKA M R R N S a Ł R H O Z R Żo EJOW LUBLIN B O IECK Palaces and Mansions; Museums P M E A
a g u ł D B hemiczna i C iego e lsk W Ch Po ł emiczn wa ojska a a o y Targ W w g i n o zafirowa n ów S irak yb A S C . a k F AL. PRZYJAŹN h o I a r s ł East of Poland Cycling Trail Green Velo e a y a TERESPOL g i c s w m z E373 z 12 u y c 816 n o ł a n i t K r c i I DOROHUSK z y - m a D o N s z A M e Churches; cloisters; Orthodox churches;w synagogues Ż Ź d na s k n N cz L a z a A Cmentarz Jeńców i a n o t ł a W o ar z oc r J T . łn O y ó d a P e Y www.greenvelo.pl web portal z p R r P Wojennych ie i Z BRZESKA M r r n s A ł R H o z R Żo EJOW LUBLIN B o IECK Palaces and mansions; museums P M e A A A K w . L S w U . ZEA P BRSK G 82 B E R L K 12 PA Z a EL E373 A A RAPMA BR s S REJ M S o KA OW RA Żytnia a IECK k A A a Z SK ł g L ł E i Hotels; tourist information centres; parking facilities B n e ą z A . U K u L ES Z ł a R t d g B iN c PA J a AM W o R o m k D R zy KA . -

Wykaz Kół Łowieckich Na Terenie Powiatu Ostrów
Wykaz kół łowieckich na terenie powiatu Ostrów numer miejscowości przynależne do powiat gmina koło łowieckie adres korespondencyjny mail Prezes Koła obwodu obwodu łowieckiego Ostrów Wielkopolski (m.) Sławin, Ołobok, Rososzyca, Sieroszewice, Parczew, Bibianki, Biskupice Ołoboczne, K.Ł. Nr 7 "BAŻANT" Ostrów Skalmierzyce, Bilczew, Psary, Moszczanka 118, 63-440 Raszków 453 [email protected] Jacek Kopecki Wlkp. Gostyczyna, Chotów, Węgry, Mączniki, Śmiłów, Osiek, Żydów, Strzegowa, Śliwniki, Sulisławice Czekanów, Młynów, Biskupice, Kościuszków, Wtórek, Czachory, Parczew, Sieroszewice, Trąba, Ostrów W.K.Ł. Nr 49 "SZARAK" Ostrów Leśnictwo Wtórek, Kąkolewo 1, 63- 452 Wlkp., Gniazdów, Fabianów, [email protected] Andrzej Perz Wlkp. 400 Ostrów Wlkp. Kęszyce, Nowe Skalmierzyce, Boczków, Trusków, Kunów, Nowe Skalmierzyce Zaborów, Miedzianów, Gałązki Małe, Kawetczyzna, Kwiatków Borowiec, Sobótka, Gutów, Gałązki Małe, Gałązki Wielkie, Droszew, Kotowiecko, K.Ł. Nr 10 "ZALESIE" Ostrów Wisławka 1, 63-430 Odolanów 449 Miedzianów, Trkusów, Głóski, [email protected] Jan Mróz Wlkp. Kościelna Wieś, Czechel, Karsy, Żychlin, Borucin, Grudzielec, Nowa Wieś Biskupice, Boczków, Chotów, Głóski, Gniazdów, Kalisz, ul. Stefana Batorego 1/14, 63-300 W.K.Ł. Nr 47 "ORĘŻ" Pleszew 391 Szczypiorno, Kościelna Wieś, [email protected] Andrzej Biesiada Pleszew Murowaniec, Skalmierzyce, Sulisławice, Trkusów, Żydów Drogosław, Walentynów, Janków Zaleśny, Pogrzybów, Przybysławice, Raszków, K.Ł. Nr 10 "ZALESIE" Ostrów Niemojewiec, Sulisław, Wisławka 1, 63-430 Odolanów 450 [email protected] Jan Mróz Wlkp. Wierzbno, Tarchały Małe, Gorzyce Małe, Radziwiłów, Gorzyce Wielkie, Lamki, Zalesie, Świeligów, Jaskółki Biadki, Janków, Zaleśny, Łąkociny, Doniszyn, Wierzbno, Gliśnica, Odolanów, Raczyce, ul. Narutowicza 4, 63-400 Ostrów Sulmierzyce, Chwaliszew, K.Ł. Nr 43 "PONOWA" Biadki 454 Jan Pabich Wlkp. Świnków, Warszty, Mazury, Cegły, Chruszczyny, Nabyszyce, Kuroch, Kaczory, Uciechów Odolanów Dębnica, Czarny Las, Ludwików, Antonin, Kozły, Antonin, ul. -

Dziennik Nr 055-2010 Pozycja 1246.Pdf
Dziennik Urzędowy Województwa Wielkopolskiego Nr 55 – 5946 – Poz. 1245, 1246 1245 UCHWAŁA Nr XLIII/451/2010 RADY MIASTA I GMINY SZAMOTUŁY z dnia 11 stycznia 2010 r. w sprawie: w sprawie wprowadzenia zmiany do Uchwały Nr XXIX/282/2008 Rady Miasta i Gminy Sza- motuły z dnia 17 listopada 2008 roku w sprawie opłaty targowej Na podstawie art. 18 ust. 2 pkt 8, art. 40 ust. §1. W uchwale Nr XXIX/282/2008 Rady Miasta i 1 ustawy z dnia 8 marca 1990 roku o samorzą- Gminy Szamotuły z dnia 17 listopada 2008 roku w dzie gminnym (t. j. Dz.U. z 2001 roku Nr 142 poz. sprawie opłaty targowej paragraf 4 ustęp 2 otrzy- 1591; z 2002 roku Nr 23 poz. 220, Nr 62 poz. 558, muje brzmienie: Nr 113 poz. 984, Nr 153 poz. 1271, Nr 214 poz. „2. Pobór opłaty targowej powierza się „Zakłado- 1806; z 2003 roku Nr 80 poz. 717, Nr 162 poz. wi Gospodarki Komunalnej w Szamotułach” Spółka 1568; z 2004 roku Nr 102 poz. 1055, Nr 116 poz. z o.o. z siedzibą w Szamotułach”, zwanemu w dal- 1203; z 2005 roku Nr 172 poz. 1441, Nr 175 poz. szym ciągu uchwały Zakładem Gospodarki Komu- 1457; z 2006 roku Nr 17 poz. 128, Nr 181 poz. nalnej w Szamotułach.” 1337; z 2007 roku Nr 48, poz. 327, Nr 138 poz. §2. Wykonanie uchwały zleca się Burmistrzowi 974, Nr 173 poz. 1218; z 2008 roku Nr 180 poz. Miasta i Gminy Szamotuły. 1111, Nr 223 poz. 1458; z 2009 roku Nr 52 poz. §3. -

Uchwała Nr XLIV/357/2018 Z Dnia 27 Marca 2018 R
DZIENNIK URZĘDOWY WOJEWÓDZTWA WIELKOPOLSKIEGO Poznań, dnia 6 kwietnia 2018 r. Poz. 3125 UCHWAŁA NR XLIV/357/2018 RADY MIEJSKIEJ W OSTROROGU z dnia 27 marca 2018 r. w sprawie podziału Miasta i Gminy Ostroróg na okręgi wyborcze, ustalenia ich granic i numerów oraz liczby radnych wybieranych w okręgu wyborczym. Na podstawie art. 12 ust. 1 ustawy z dnia 11 stycznia 2018 r. o zmianie niektórych ustaw w celu zwiększenia udziału obywateli w procesie wybierania, funkcjonowania i kontrolowania niektórych organów publicznych (Dz. U. z 2018 r., poz. 130) w związku z art. 418 § 1, art. 419 § 2 i § 4 oraz art. 420 § 1 ustawy z dnia 5 stycznia 2011 r. Kodeks wyborczy (t.jedn. Dz. U. z 2017 r., poz. 15 ze zmianami), Rada Miejska w Ostrorogu uchwala, co następuje: § 1. Dla wyboru Rady Miejskiej w Ostrorogu dokonuje się podziału Gminy Ostroróg na okręgi wyborcze, ustala się granice i numery oraz liczbę radnych wybieranych w każdym okręgu wyborczym w sposób określony w załączniku do niniejszej uchwały. § 2. Wykonanie uchwały powierza się Burmistrzowi Miasta i Gminy Ostroróg. § 3. Traci moc uchwała Nr XXI/121/2012 Rady Miejskiej w Ostrorogu z dnia 25 września 2012 r. w sprawie podziału Miasta i Gminy Ostroróg na okręgi wyborcze, ustalenia ich granic i numerów oraz liczby radnych wybieranych w okręgu wyborczym. § 4. Uchwała wchodzi w życie z dniem podjęcia i podlega ogłoszeniu w Dzienniku Urzędowym Województwa Wielkopolskiego oraz podaniu do publicznej wiadomości w sposób zwyczajowo przyjęty. § 5. Uchwałę przekazuje się niezwłocznie Wojewodzie Wielkopolskiemu w Poznaniu i Komisarzowi Wyborczemu w Pile. § 6. Wyborcom, w liczbie co najmniej 15, przysługuje prawo wniesienia skargi do Komisarza Wyborczego w Pile w terminie 5 dni od daty podania uchwały do publicznej wiadomości.