Based Vaccination and Nonviral Cytokine Gene Transfer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluation of T-Cell and B-Cell Epitopes and Design of Multivalent Vaccines Against Htlv-1 Diseases
EVALUATION OF T-CELL AND B-CELL EPITOPES AND DESIGN OF MULTIVALENT VACCINES AGAINST HTLV-1 DISEASES DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Roshni Sundaram, M.S. * * * * * The Ohio State University 2003 Dissertation Committee: Approved by Professor Pravin T.P. Kaumaya, Adviser Professor Christopher M. Walker Adviser Professor Neil R. Baker Department of Microbiology Professor Marshall V. Williams ABSTRACT Human T-cell lymphotropic virus type I (HTLV-1) is a C type retrovirus that is the causative agent of an aggressive T-cell malignancy, adult T-cell leukemia/lymphoma (ATLL). The virus is also implicated in a number of inflammatory disorders, the most prominent among them being HTLV-1 associated myelopathy or tropical spastic paraparesis (HAM/TSP). HTLV-1, like many viruses that cause chronic infection, has adapted to persist in the face of an active immune response in infected individuals. The viral transactivator Tax is the primary target of the cellular immune response and humoral responses are mainly directed against the envelope protein. Vaccination against HTLV-1 is a feasible option as there is very little genetic and antigenic variability. Vaccination regimes against chronic viruses must be aimed at augmenting the immune response to a level that is sufficient to clear the virus. This requires that the vaccine delivers a potent stimulus to the immune system that closely resembles natural infection to activate both the humoral arm and the cellular arm. It is also clear that multicomponent vaccines may be more beneficial in terms of increasing the breadth of the immune response as well as being applicable in an outbred population. -

Monoclonal Antibody to Macrophages (Haematopoiesis Associated) - FITC
OriGene Technologies, Inc. OriGene Technologies GmbH 9620 Medical Center Drive, Ste 200 Schillerstr. 5 Rockville, MD 20850 32052 Herford UNITED STATES GERMANY Phone: +1-888-267-4436 Phone: +49-5221-34606-0 Fax: +1-301-340-8606 Fax: +49-5221-34606-11 [email protected] [email protected] BM4022F Monoclonal Antibody to Macrophages (Haematopoiesis associated) - FITC Alternate names: Macrophage marker Quantity: 0.2 mg Concentration: 0.4 mg/ml Background: 25F9 is associated with well-differentiated tissue macrophages both in normal and diseased tissues independently of the presence or absence of inflammation. The antigen is a 86kDa membrane protein, the epitope has not been further characterized. Host / Isotype: Mouse / IgG1 Recommended Isotype SM10F (for use in human samples) Controls: Clone: 25F9 Immunogen: Cultured human monocytes. Format: State: Liquid purified Ig fraction. Purification: Affinity chromatography. Buffer System: PBS buffer pH 7.2 with 0.09% sodium azide as preservative and 10 mg/ml BSA as stabilizer. Label: FITC Applications: Has been described to work in FACS. Antigen Distribution on Isolated cells and Tissue sections: Absent on freshly isolated monocytes and other blood cells; present on 40 - 50% of human monocytes after 6-7 day culture, also positive on some melanoma and carcinoma cell lines. Kupffer cells, histiocytes (skin), macrophages of the thymus, in the germinal centres of lymph nodes and spleen, in mamma carcinoma, melanoma, osteocarcinoma and gastric cancer; excema, sarcoidosis, BCG granuloma; synovial lining cells, tuberculoid leprosy: no expression in lepramatous leprosy. Other markers also used in the above studies include RM3/1, 27E10, G16/1, S36.48 and 8-5C2 in various combinations. -

Theory of an Immune System Retrovirus
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 9159-9163, December 1986 Medical Sciences Theory of an immune system retrovirus (human immunodeficiency virus/acquired immune deficiency syndrome) LEON N COOPER Physics Department and Center for Neural Science, Brown University, Providence, RI 02912 Contributed by Leon N Cooper, July 23, 1986 ABSTRACT Human immunodeficiency virus (HIV; for- initiates clonal expansion, sustained by interleukin 2 and y merly known as human T-cell lymphotropic virus type interferon. Ill/lymphadenopathy-associated virus, HTLV-Ill/LAV), the I first give a brief sketch of these events in a linked- retrovirus that infects T4-positive (helper) T cells of the interaction model in which it is assumed that antigen-specific immune system, has been implicated as the agent responsible T cells must interact with the B-cell-processed virus to for the acquired immune deficiency syndrome. In this paper, initiate clonal expansion (2). I then assume that virus-specific I contrast the growth of a "normal" virus with what I call an antibody is the major component ofimmune system response immune system retrovirus: a retrovirus that attacks the T4- that limits virus spread. As will be seen, the details of these positive T cells of the immune system. I show that remarkable assumptions do not affect the qualitative features of my interactions with other infections as well as strong virus conclusions. concentration dependence are general properties of immune Linked-Interaction Model for Clonal Expansion of Lympho- system retroviruses. Some of the consequences of these ideas cytes. Let X be the concentration of normal infecting virus are compared with observations. -

Difference Between Antigen and Immunogen What Is an Antigen?
Difference Between Antigen and Immunogen www.differencebetween.com Key Difference - Antigen vs Immunogen Immunology is a branch of medicine and biology and is concerned about all aspects of the immune system in organisms. This is a much studied as it is vital to identify and assess the manner in which an organism protects itself against a foreign invasion. An immunological response is initiated upon the entry of a foreign entity which results in a cascade of reactions downstream to degrade or eliminate the foreign entity. An antigen is a foreign body or a molecule, which has the ability to bind to the antibody produced by the host in response to the recognition of the antigen. An immunogen is also a foreign molecule which can elicit an immune response by triggering the host immune system. The key difference between the antigen and the immunogen is their ability and the inability to generate an immune response;an immunogen is necessarily an antigen, but an antigen may not necessarily be an immunogen. What is an Antigen? Antigens are small molecular recognition sites present in the cellular surface of many bacteria, fungi, viruses, dust particles and other cellular and noncellular particles. These small molecules referred to as antigens,and they can be recognized by the host immune system. Antigens are mainly composed of proteins, amino acids, lipids, glycolipids, glycoproteins or nucleic acid markers. These molecules possess the ability to bind to the antibodies (immunoglobulins produced by B cells) produced by the host as an immune response. Antigens are also capable of triggering the host immune system to initiate an immune mechanism. -

Cells Through Regulation of Follicular Helper T B7RP-1 Blockade
The Journal of Immunology B7RP-1 Blockade Ameliorates Autoimmunity through Regulation of Follicular Helper T Cells Yi-Ling Hu,* Daniela P. Metz,* James Chung,† Gerald Siu,1 and Ming Zhang2* Autoimmune diseases are marked by the presence of class-switched, high-affinity autoantibodies with pathogenic potential. Co- stimulation plays an important role in the activation of T cells and the development of T cell-dependent B cell responses. ICOS plays an indispensable role in the development of follicular helper T cells (TFH cells), which provide cognate help to germinal center (GC) B cells. We show that the levels of TFH cells and GC B cells in two different models of autoimmunity, the New Zealand Black/New Zealand White (NZB/NZW) F1 mouse model of systemic lupus erythematosus and the collagen-induced arthritis model of rheumatoid arthritis, are dependent on the maintenance of the ICOS/B7RP-1 pathway. Treatment with an anti-B7RP-1 Ab ameliorates disease manifestations and leads to a decrease in TFH cells and GC B cells as well as an overall decrease in the frequency of ICOS؉ T cells. Coculture experiments of Ag-primed B cells with CXCR5؉ or CXCR5؊ T cells show that blocking B7RP-1 does not directly impact the production of IgG by B cells. These findings further support the role of ICOS in autoimmunity and suggest that the expansion of the TFH cell pool is an important mechanism by which ICOS regulates Ab production. The Journal of Immunology, 2009, 182: 1421–1428. ffective activation of T cells via their Ag receptor requires ing anti-B7RP-1 reagents effectively inhibited the initiation and additional stimuli provided by cell-surface costimulatory maturation of an Ab-mediated Ag response (15). -

Influence of Vitamin C on Lymphocytes: an Overview
antioxidants Review Influence of Vitamin C on Lymphocytes: An Overview Gwendolyn N. Y. van Gorkom 1,*, Roel G. J. Klein Wolterink 1, Catharina H. M. J. Van Elssen 1 ID , Lotte Wieten 2, Wilfred T. V. Germeraad 1 and Gerard M. J. Bos 1 1 Division of Hematology, Department of Internal Medicine, GROW-School for Oncology and Developmental Biology, Maastricht University Medical Center, 6202AZ Maastricht, The Netherlands; [email protected] (R.G.J.K.W.); [email protected] (C.H.M.J.V.E.); [email protected] (W.T.V.G.); [email protected] (G.M.J.B.) 2 Department of Transplantation Immunology, Maastricht University Medical Center, 6202 AZ Maastricht, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +31-043-387-6543 Received: 8 February 2018; Accepted: 8 March 2018; Published: 10 March 2018 Abstract: Vitamin C or ascorbic acid (AA) is implicated in many biological processes and has been proposed as a supplement for various conditions, including cancer. In this review, we discuss the effects of AA on the development and function of lymphocytes. This is important in the light of cancer treatment, as the immune system needs to regenerate following chemotherapy or stem cell transplantation, while cancer patients are often AA-deficient. We focus on lymphocytes, as these white blood cells are the slowest to restore, rendering patients susceptible to often lethal infections. T lymphocytes mediate cellular immunity and have been most extensively studied in the context of AA biology. In vitro studies demonstrate that T cell development requires AA, while AA also enhances T cell proliferation and may influence T cell function. -

Vaccine Immunology Claire-Anne Siegrist
2 Vaccine Immunology Claire-Anne Siegrist To generate vaccine-mediated protection is a complex chal- non–antigen-specifc responses possibly leading to allergy, lenge. Currently available vaccines have largely been devel- autoimmunity, or even premature death—are being raised. oped empirically, with little or no understanding of how they Certain “off-targets effects” of vaccines have also been recog- activate the immune system. Their early protective effcacy is nized and call for studies to quantify their impact and identify primarily conferred by the induction of antigen-specifc anti- the mechanisms at play. The objective of this chapter is to bodies (Box 2.1). However, there is more to antibody- extract from the complex and rapidly evolving feld of immu- mediated protection than the peak of vaccine-induced nology the main concepts that are useful to better address antibody titers. The quality of such antibodies (e.g., their these important questions. avidity, specifcity, or neutralizing capacity) has been identi- fed as a determining factor in effcacy. Long-term protection HOW DO VACCINES MEDIATE PROTECTION? requires the persistence of vaccine antibodies above protective thresholds and/or the maintenance of immune memory cells Vaccines protect by inducing effector mechanisms (cells or capable of rapid and effective reactivation with subsequent molecules) capable of rapidly controlling replicating patho- microbial exposure. The determinants of immune memory gens or inactivating their toxic components. Vaccine-induced induction, as well as the relative contribution of persisting immune effectors (Table 2.1) are essentially antibodies— antibodies and of immune memory to protection against spe- produced by B lymphocytes—capable of binding specifcally cifc diseases, are essential parameters of long-term vaccine to a toxin or a pathogen.2 Other potential effectors are cyto- effcacy. -

Immunogen Design
applications and troubleshooting appendix chapter 21 Immunogen Design Antigens Used for Antibody Production • You can use synthetic peptides, recombinant proteins, immunogens, and others. We do not recommend the use of whole cells for anti- body production. • Arbitrary selection of the antigenic peptide or use of a recombinant fusion protein usually will result in a 65-70% success rate using standard methodologies. If the antigenic peptide is chosen using standard bioinformatic algorithms, you can increase your success rate to 75-80%. If this optimized peptide is then conjugated using Invitrogen’s proprietary approach, you can increase your success rate to about 85-90%. A recent collaborative study showed that approximately 450 polyclonal peptide antibodies out of 500 individual protein targets recognized their target by western blot analysis. Peptide Conjugation • Peptides are normally too short to be immunogenic. A carrier protein enhances immunogenicity. MAP and KLH are the two most popular conjugation methods. Other available conjugates are OVA (Ovalbumin), BSA (Bovine Serum Albumin), and THY (Thyroglobulin). • Conjugation is usually done through the sulfhydryl group of a Cys designed into the peptide. • For peptides < 6 kDa, conjugation will be required. • If studying invertebrate species, you should use carrier proteins such as ovalbumin and KLH (from sea snail). KLH has no homology to 21.2 vertebrate proteins. Invitrogen utilizes a proprietary multiple conjugation method where we conjugate peptides through both termini and through key internal sites. This methodology presents the peptides in alternate, multiple conformations, making them more antigenic and producing antibodies appendix with greater utility across assays. Our conjugation scheme provides a better matching of epitopes between the presented peptide and the way the peptides are presented in the native protein. -
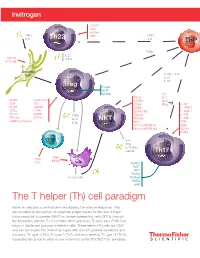
Cell Paradigm
CCR10 CD4 PDGFR TNFα Th22 TCR TNFα I L- 6 I L- 6 ThP TGFβ I L-2 2 CD11C TNFα DEC205 Dendritic cell TGFβ + IL-6 I L-21 I L-23 Treg Foxp3 AHR STAT5 I L-2 CD25 I L- 4 CD25 GITR/AITR CD49b IL-27 CD4 TCR CD69 IFNγ T I L-1R1 CD39 CXCR3 CD94 I L-12R B1 CD73 CCR4 CD160 (m) I L-13 R α1 CD101 CCR9 TGFβ CD244 I L-21R FR4 (m) CTLA-4 I L-10 NKT NKG2D I L-23 R GARP (activated) IL-35 NK1.1 TCR Vα24Jα18TCR (h) CD4 Vα14Jα18TCR (m) CD161 CCR6 Effector T I L- 4 IFNγ I L-17 ( i N K T subset) Th17 MHCI APC TCR MHCII CD3 RUNX1 BATF IRF4 RORγt I L-15 / I L-15 R RORα4 STAT3 AHR The T helper (Th) cell paradigm Naive Th cells play a central role in modulating the immune response. They are activated by recognition of a peptide antigen bound to the class II major histocompatibility complex (MHC) on antigen-presenting cells (APCs) through the interaction with the T cell receptor. After activation, Th precursor (ThP) cells begin to divide and give rise to effector cells. These effector Th cells are CD4⁺ and can be divided into three main types with distinct cytokine secretions and functions: Th type 1 (Th1), Th type 2 (Th2), and most recently Th type 17 (Th17), expanding the group to what is now referred to as the Th1/Th2/Th17 paradigm. The T helper (Th) cell paradigm DEC205 Dendritic CD11c cell CCR10 CD4 CD4 PDGFR TCR TNFα Th22 TCR TNFα Antigen presentation I L- 6 I L- 6 ThP Costimulatory signals TGFβ Th9 I L- 4 TGFβ I L-2 2 CD11C I L- 9 TNFα I L-2 I L-25 DEC205 I L- 4 IL-31 I L-15 ( h) IL-33 TCR CD4 Dendritic CD30 cell TGFβ + IL-6 I L-21 CCR8 I L-23 I L- 6 CXCR4 -

Harnessing the Plasticity of CD4+ T Cells to Treat Immune-Mediated Disease
REVIEWS Harnessing the plasticity of CD4+ T cells to treat immune-mediated disease Michel DuPage and Jeffrey A. Bluestone Abstract | CD4+ T cells differentiate and acquire distinct functions to combat specific pathogens but can also adapt their functions in response to changing circumstances. Although this phenotypic plasticity can be potentially deleterious, driving immune pathology, it also provides important benefits that have led to its evolutionary preservation. Here, we review CD4+ T cell plasticity by examining the molecular mechanisms that regulate it — from the extracellular cues that initiate and drive cells towards varying phenotypes, to the cytosolic signalling cascades that decipher these cues and transmit them into the cell and to the nucleus, where these signals imprint specific gene expression programmes. By understanding how this functional flexibility is achieved, we may open doors to new therapeutic approaches that harness this property of T cells. The hypothesis of T helper 1 (TH1) and TH2 cell subsets Thus, there has been a re‑emergence of T cell ‘plas‑ championed by Mosmann and Coffman in the 1980s, ticity’, as opposed to ‘lineage stability’, as the evolving and others since, provided a framework to understand paradigm12 (BOX 1). how CD4+ T cells direct diverse immune responses1,2. In this Review, we summarize a rapidly expanding By examining clonal populations of CD4+ T cells, they literature surrounding CD4+ T cell subsets and make found that different clones expressed selected pat‑ the case for broad plasticity -

T Helper Cell Differentiation: Understanding the Needs of Hierarchy
Immunity Previews T Helper Cell Differentiation: Understanding the Needs of Hierarchy Thomas Weichhart1,* and Marcus D. Sa¨ emann1,* 1Department of Internal Medicine III, Clinical Division of Nephrology and Dialysis, Medical University Vienna, Wa¨ hringer Gu¨ rtel 18-20, A-1090 Vienna, Austria *Correspondence: [email protected] (T.W.), [email protected] (M.D.S.) DOI 10.1016/j.immuni.2010.06.008 In this issue of Immunity, Lee et al. (2010) demonstrate that the mammalian Target of Rapamycin Complex 2 promotes the differentiation of T helper 1 (Th1) cells via the kinase Akt, whereas it independently fosters Th2 cell generation via another kinase, PKC-q. Regulation of the manifold and flexible mTOR is a critical regulator of memory Akt S473 was defective after stimulation requirements of an efficient immune CD8+ T cell generation as well as inflam- with CD3 and CD28 mAb, whereas response to eliminate dangerous micro- matory responses in myeloid antigen-pre- mTORC1-mediated phosphorylation of bes depends on a delicate balance of senting cells (Araki et al., 2009; Weichhart S6K1 was intact. Functionally, however, diverse T helper (Th) cell subsets (Zhu et al., 2008). mTOR is the core component it was found that Rictor-deficient CD4+ et al., 2010). Hence, naive CD4+ Th cells of mTORC1 (mTOR complex 1), which is T cells were unable to differentiate into differentiate into polarized effector Th composed by the adaptor protein Raptor, both Th1 or Th2 cells in vitro, demon- cell subsets depending on the priming whereas Rictor and Sin1 classify strating that mTORC2 is vital for Th1 and cytokine milieu. -

6229 T Cell Regulation of Hematopoiesis Alexander L Dent1
[Frontiers in Bioscience 13, 6229-6236, May 1, 2008] T cell regulation of hematopoiesis Alexander L Dent1, Mark H. Kaplan1,2 1Department of Microbiology and Immunology and Walther Oncology Center, Indiana University School of Medicine, Indianapolis, IN 46202, 2Department of Pediatrics, Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202 TABLE OF CONTENTS 1. Abstract 2. Introduction 3. T cells are required for myeloid cell maturation 4. Th1 cells regulate Hematopoietic Progenitor Cell homeostasis 5. The role of Th2 cells in modulating hematopoiesis 6. IL-17-secreting “Th17” cells regulate peripheral neutrophil numbers 7. Natural killer T cell regulate HSC and HPC activity 8. CD8 cells can alter hematopoietic progenitor cell activity. 9. Do Regulatory T cells influence hematopoiesis? 10. Perspective 11. References 1. ABSTRACT 2. INTRODUCTION It has long been known that thymus-derived lymphocytes (T cells) can produce cytokines that have T cells secrete several well-known cytokines, powerful effects on hematopoiesis. All major classes of T such as IL-3, IL-5, IL-6, GM-CSF and M-CSF that strongly cells-- CD4 T helper cells, CD4 regulatory T cells, CD8 T influence and promote hematopoietic cell development. cells, γδ T cells and NKT cells-- produce a number of These cytokines act on hematopoiesis through mechanisms cytokines and chemokines that can modulate that are both direct and indirect (1-3). Each of these hematopoiesis. More recent research has shown that cytokines can potentially regulate hematopoiesis at several specific T helper cell types, such as Th1, Th2 and Th17 stages, including the regulation of hematopoietic stem cells cells, with the development of each subset depending on (HSC), hematopoietic progenitor cells (HPC) and mature distinct STAT proteins, have the potential to modulate the cells in the periphery.