From Molecular Phylogenetics to the Evolution of Life History And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Colorado Wildlife Action Plan: Proposed Rare Plant Addendum
Colorado Wildlife Action Plan: Proposed Rare Plant Addendum By Colorado Natural Heritage Program For The Colorado Rare Plant Conservation Initiative June 2011 Colorado Wildlife Action Plan: Proposed Rare Plant Addendum Colorado Rare Plant Conservation Initiative Members David Anderson, Colorado Natural Heritage Program (CNHP) Rob Billerbeck, Colorado Natural Areas Program (CNAP) Leo P. Bruederle, University of Colorado Denver (UCD) Lynn Cleveland, Colorado Federation of Garden Clubs (CFGC) Carol Dawson, Bureau of Land Management (BLM) Michelle DePrenger-Levin, Denver Botanic Gardens (DBG) Brian Elliott, Environmental Consulting Mo Ewing, Colorado Open Lands (COL) Tom Grant, Colorado State University (CSU) Jill Handwerk, Colorado Natural Heritage Program (CNHP) Tim Hogan, University of Colorado Herbarium (COLO) Steve Kettler, U.S. Fish and Wildlife Service (USFWS) Andrew Kratz, U.S. Forest Service (USFS) Sarada Krishnan, Colorado Native Plant Society (CoNPS), Denver Botanic Gardens Brian Kurzel, Colorado Natural Areas Program Eric Lane, Colorado Department of Agriculture (CDA) Paige Lewis, The Nature Conservancy (TNC) Ellen Mayo, U.S. Fish and Wildlife Service Mitchell McGlaughlin, University of Northern Colorado (UNC) Jennifer Neale, Denver Botanic Gardens Betsy Neely, The Nature Conservancy Ann Oliver, The Nature Conservancy Steve Olson, U.S. Forest Service Susan Spackman Panjabi, Colorado Natural Heritage Program Jeff Peterson, Colorado Department of Transportation (CDOT) Josh Pollock, Center for Native Ecosystems (CNE) Nicola Ripley, -

Appendix F3 Rare Plant Survey Report
Appendix F3 Rare Plant Survey Report Draft CADIZ VALLEY WATER CONSERVATION, RECOVERY, AND STORAGE PROJECT Rare Plant Survey Report Prepared for May 2011 Santa Margarita Water District Draft CADIZ VALLEY WATER CONSERVATION, RECOVERY, AND STORAGE PROJECT Rare Plant Survey Report Prepared for May 2011 Santa Margarita Water District 626 Wilshire Boulevard Suite 1100 Los Angeles, CA 90017 213.599.4300 www.esassoc.com Oakland Olympia Petaluma Portland Sacramento San Diego San Francisco Seattle Tampa Woodland Hills D210324 TABLE OF CONTENTS Cadiz Valley Water Conservation, Recovery, and Storage Project: Rare Plant Survey Report Page Summary ............................................................................................................................... 1 Introduction ..........................................................................................................................2 Objective .......................................................................................................................... 2 Project Location and Description .....................................................................................2 Setting ................................................................................................................................... 5 Climate ............................................................................................................................. 5 Topography and Soils ......................................................................................................5 -

CDFG Natural Communities List
Department of Fish and Game Biogeographic Data Branch The Vegetation Classification and Mapping Program List of California Terrestrial Natural Communities Recognized by The California Natural Diversity Database September 2003 Edition Introduction: This document supersedes all other lists of terrestrial natural communities developed by the Natural Diversity Database (CNDDB). It is based on the classification put forth in “A Manual of California Vegetation” (Sawyer and Keeler-Wolf 1995 and upcoming new edition). However, it is structured to be compatible with previous CNDDB lists (e.g., Holland 1986). For those familiar with the Holland numerical coding system you will see a general similarity in the upper levels of the hierarchy. You will also see a greater detail at the lower levels of the hierarchy. The numbering system has been modified to incorporate this richer detail. Decimal points have been added to separate major groupings and two additional digits have been added to encompass the finest hierarchal detail. One of the objectives of the Manual of California Vegetation (MCV) was to apply a uniform hierarchical structure to the State’s vegetation types. Quantifiable classification rules were established to define the major floristic groups, called alliances and associations in the National Vegetation Classification (Grossman et al. 1998). In this document, the alliance level is denoted in the center triplet of the coding system and the associations in the right hand pair of numbers to the left of the final decimal. The numbers of the alliance in the center triplet attempt to denote relationships in floristic similarity. For example, the Chamise-Eastwood Manzanita alliance (37.106.00) is more closely related to the Chamise- Cupleaf Ceanothus alliance (37.105.00) than it is to the Chaparral Whitethorn alliance (37.205.00). -

Mcclinton Unr 0139M 13052.Pdf
University of Nevada, Reno Habitat preferences, intraspecific variation, and restoration of a rare soil specialist in northern Nevada A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Natural Resources and Environmental Science by Jamey D. McClinton Dr. Elizabeth A. Leger/Thesis Advisor December, 2019 Copyright by Jamey D. McClinton 2019 All Rights Reserved We recommend that the thesis prepared under our supervision by Jamey D. McClinton Entitled Habitat preferences, intraspecific variation, and restoration of a rare soil specialist in northern Nevada be accepted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Elizabeth Leger, Ph.D., Advisor Paul Verburg, Ph.D., Committee member Thomas Parchman, Ph.D., Graduate School Representative David W. Zeh, Ph.D., Dean, Graduate School December-2019 i Abstract Edaphic specialization in plants is associated with the development of novel adaptations that frequently lead to speciation, causing unique edaphic environments to be associated with rare and endemic plant species worldwide. These species contribute significantly to global biodiversity, but are especially vulnerable to disturbance and climate change because of their inherently patchy distributions and locally adapted populations. Successful conservation of these species depends upon understanding their habitat requirements and the amounts and distributions of genetic and phenotypic diversity among populations. Little is known about the habitat requirements or -

December 2012 Number 1
Calochortiana December 2012 Number 1 December 2012 Number 1 CONTENTS Proceedings of the Fifth South- western Rare and Endangered Plant Conference Calochortiana, a new publication of the Utah Native Plant Society . 3 The Fifth Southwestern Rare and En- dangered Plant Conference, Salt Lake City, Utah, March 2009 . 3 Abstracts of presentations and posters not submitted for the proceedings . 4 Southwestern cienegas: Rare habitats for endangered wetland plants. Robert Sivinski . 17 A new look at ranking plant rarity for conservation purposes, with an em- phasis on the flora of the American Southwest. John R. Spence . 25 The contribution of Cedar Breaks Na- tional Monument to the conservation of vascular plant diversity in Utah. Walter Fertig and Douglas N. Rey- nolds . 35 Studying the seed bank dynamics of rare plants. Susan Meyer . 46 East meets west: Rare desert Alliums in Arizona. John L. Anderson . 56 Calochortus nuttallii (Sego lily), Spatial patterns of endemic plant spe- state flower of Utah. By Kaye cies of the Colorado Plateau. Crystal Thorne. Krause . 63 Continued on page 2 Copyright 2012 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Utah Native Plant Society, PO Box 520041, Salt Lake Copyright 2012 Utah Native Plant Society. All Rights City, Utah, 84152-0041. www.unps.org Reserved. Calochortiana is a publication of the Utah Native Plant Society, a 501(c)(3) not-for-profit organi- Editor: Walter Fertig ([email protected]), zation dedicated to conserving and promoting steward- Editorial Committee: Walter Fertig, Mindy Wheeler, ship of our native plants. Leila Shultz, and Susan Meyer CONTENTS, continued Biogeography of rare plants of the Ash Meadows National Wildlife Refuge, Nevada. -

The Vascular Flora of the Upper Santa Ana River Watershed, San Bernardino Mountains, California
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/281748553 THE VASCULAR FLORA OF THE UPPER SANTA ANA RIVER WATERSHED, SAN BERNARDINO MOUNTAINS, CALIFORNIA Article · January 2013 CITATIONS READS 0 28 6 authors, including: Naomi S. Fraga Thomas Stoughton Rancho Santa Ana B… Plymouth State Univ… 8 PUBLICATIONS 14 3 PUBLICATIONS 0 CITATIONS CITATIONS SEE PROFILE SEE PROFILE Available from: Thomas Stoughton Retrieved on: 24 November 2016 Crossosoma 37(1&2), 2011 9 THE VASCULAR FLORA OF THE UPPER SANTA ANA RIVER WATERSHED, SAN BERNARDINO MOUNTAINS, CALIFORNIA Naomi S. Fraga, LeRoy Gross, Duncan Bell, Orlando Mistretta, Justin Wood1, and Tommy Stoughton Rancho Santa Ana Botanic Garden 1500 North College Avenue Claremont, California 91711 1Aspen Environmental Group, 201 North First Avenue, Suite 102, Upland, California 91786 [email protected] All Photos by Naomi S. Fraga ABSTRACT: We present an annotated catalogue of the vascular flora of the upper Santa Ana River watershed, in the southern San Bernardino Mountains, in southern California. The catalogue is based on a floristic study, undertaken from 2008 to 2010. Approximately 65 team days were spent in the field and over 5,000 collections were made over the course of the study. The study area is ca. 155 km2 in area (40,000 ac) and ranges in elevation from 1402 m to 3033 m. The study area is botanically diverse with more than 750 taxa documented, including 56 taxa of conservation concern and 81 non-native taxa. Vegetation and habitat types in the area include chaparral, evergreen oak forest and woodland, riparian forest, coniferous forest, montane meadow, and pebble plain habitats. -

Restoration Fremontia Vol
VOL. 48, NO.1 NOVEMBER 2020 RESTORATION FREMONTIA VOL. 48, NO.1, NOVEMBER 2020 FROM THE EDITORS What kind of world do we want, and how do we get there? These are Protecting California’s native flora since the questions that drive restoration, the central theme of this issue. They 1965 are also the questions that have led the California Native Plant Society Our mission is to conserve California’s native leadership to initiate an important change to this publication, which will plants and their natural habitats, and increase take effect in the spring 2021 issue. understanding, appreciation, and horticultural The name of this publication, Fremontia, has been a point of concern use of native plants. and discussion since last winter, when members of the CNPS leader- ship learned some disturbing facts about John C. Frémont, from whom Copyright ©2020 dozens of North American plants, including the flannelbush plant California Native Plant Society Fremontodendron californicum, derive their names. According to multi- ISSN 0092-1793 (print) ple sources, including the State of California Native American Heritage ISSN 2572-6870 (online) Commission, Frémont was responsible for brutal massacres of Native Americans in the Sacramento Valley and Klamath Lake. As a consequence, The views expressed by the authors in this issue do not necessarily represent policy or proce- the CNPS board of directors voted unanimously to rename Fremontia, a dure of CNPS. process slated for completion by the end of 2020. The decision to rename Fremontia, a name that dates back to the ori- gins of the publication in 1973, is about the people who have been—and 2707 K Street, Suite 1 continue to be—systematically excluded from the conservation commu- Sacramento, CA 95816-5130 nity. -
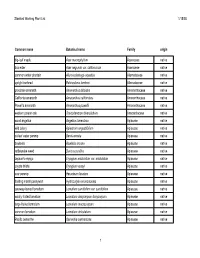
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -

Fremontia Journal of the California Native Plant Society
$10.00 (Free to Members) VOL. 40, NO. 3 AND VOL. 41, NO. 1 • SEPTEMBER 2012 AND JANUARY 2013 FREMONTIA JOURNAL OF THE CALIFORNIA NATIVE PLANT SOCIETY INSPIRATIONINSPIRATION ANDAND ADVICEADVICE FOR GARDENING VOL. 40, NO. 3 AND VOL. 41, NO. 1, SEPTEMBER 2012 AND JANUARY 2013 FREMONTIA WITH NATIVE PLANTS CALIFORNIA NATIVE PLANT SOCIETY CNPS, 2707 K Street, Suite 1; Sacramento, CA 95816-5130 FREMONTIA Phone: (916) 447-CNPS (2677) Fax: (916) 447-2727 Web site: www.cnps.org Email: [email protected] VOL. 40, NO. 3, SEPTEMBER 2012 AND VOL. 41, NO. 1, JANUARY 2013 MEMBERSHIP Membership form located on inside back cover; Copyright © 2013 dues include subscriptions to Fremontia and the CNPS Bulletin California Native Plant Society Mariposa Lily . $1,500 Family or Group . $75 Bob Hass, Editor Benefactor . $600 International or Library . $75 Rob Moore, Contributing Editor Patron . $300 Individual . $45 Plant Lover . $100 Student/Retired/Limited Income . $25 Beth Hansen-Winter, Designer Cynthia Powell, Cynthia Roye, and CORPORATE/ORGANIZATIONAL Mary Ann Showers, Proofreaders 10+ Employees . $2,500 4-6 Employees . $500 7-10 Employees . $1,000 1-3 Employees . $150 CALIFORNIA NATIVE STAFF – SACRAMENTO CHAPTER COUNCIL PLANT SOCIETY Executive Director: Dan Gluesenkamp David Magney (Chair); Larry Levine Finance and Administration (Vice Chair); Marty Foltyn (Secretary) Dedicated to the Preservation of Manager: Cari Porter Alta Peak (Tulare): Joan Stewart the California Native Flora Membership and Development Bristlecone (Inyo-Mono): Coordinator: Stacey Flowerdew The California Native Plant Society Steve McLaughlin Conservation Program Director: Channel Islands: David Magney (CNPS) is a statewide nonprofit organi- Greg Suba zation dedicated to increasing the Rare Plant Botanist: Aaron Sims Dorothy King Young (Mendocino/ understanding and appreciation of Vegetation Program Director: Sonoma Coast): Nancy Morin California’s native plants, and to pre- Julie Evens East Bay: Bill Hunt serving them and their natural habitats Vegetation Ecologists: El Dorado: Sue Britting for future generations. -

Rare Plant Survey of San Juan Public Lands, Colorado
Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 Rare Plant Survey of San Juan Public Lands, Colorado 2005 Prepared by Peggy Lyon and Julia Hanson Colorado Natural Heritage Program 254 General Services Building Colorado State University Fort Collins CO 80523 December 2005 Cover: Imperiled (G1 and G2) plants of the San Juan Public Lands, top left to bottom right: Lesquerella pruinosa, Draba graminea, Cryptantha gypsophila, Machaeranthera coloradoensis, Astragalus naturitensis, Physaria pulvinata, Ipomopsis polyantha, Townsendia glabella, Townsendia rothrockii. Executive Summary This survey was a continuation of several years of rare plant survey on San Juan Public Lands. Funding for the project was provided by San Juan National Forest and the San Juan Resource Area of the Bureau of Land Management. Previous rare plant surveys on San Juan Public Lands by CNHP were conducted in conjunction with county wide surveys of La Plata, Archuleta, San Juan and San Miguel counties, with partial funding from Great Outdoors Colorado (GOCO); and in 2004, public lands only in Dolores and Montezuma counties, funded entirely by the San Juan Public Lands. Funding for 2005 was again provided by San Juan Public Lands. The primary emphases for field work in 2005 were: 1. revisit and update information on rare plant occurrences of agency sensitive species in the Colorado Natural Heritage Program (CNHP) database that were last observed prior to 2000, in order to have the most current information available for informing the revision of the Resource Management Plan for the San Juan Public Lands (BLM and San Juan National Forest); 2. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Current Tracking List
Nevada Division of Natural Heritage Department of Conservation and Natural Resources 901 S. Stewart Street, Suite 5002, Carson City, Nevada 89701-5245 voice: (775) 684-2900 | fax: (775) 684-2909 | web: heritage.nv.gov At-Risk Plant and Animal Tracking List July 2021 The Nevada Division of Natural Heritage (NDNH) A separate list, the Plant and Animal Watch List, systematically curates information on Nevada's contains taxa that could become at-risk in the future. endangered, threatened, sensitive, rare, and at-risk plants and animals providing the most comprehensive Taxa on the At-Risk Plant and Animal Tracking List are source of information on Nevada’s imperiled organized by taxonomic group, and presented biodiversity. alphabetically by scientific name within each group. Currently, there are 639 Tracking List taxa: 285 plants, Nevada's health and economic well-being depend 209 invertebrates, 65 fishes, 9 amphibians, 7 reptiles, upon its biodiversity and wise land stewardship. This 27 birds, and 37 mammals. challenge increases as population and land-use pressures continue to grow. Nevada is among the top Documentation of population status, locations, or 10 states for both the diversity and the vulnerability of other updates or corrections for any of the taxa on its living heritage. With early planning and responsible this list are always welcome. Literature citations with development, economic growth and our biological taxonomic revisions and descriptions of new taxa are resources can coexist. NDNH is a central source for also appreciated. The Nevada Native Species Site information critical to achieving this balance. Survey Report form is available on our website under Management priorities for the state’s imperiled the Submit Data tab and is the preferred format for biodiversity are continually assessed, providing submitting information to NDNH.