Spatio-Temporal Patterns of Introduced Mice and Invertebrates on Antipodes Island
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proposal for Inclusion of the Antipodean Albatross in Appendix I
CONVENTION ON UNEP/CMS/COP13/Doc. 27.1.7 MIGRATORY 25 September 2019 Original: English SPECIES 13th MEETING OF THE CONFERENCE OF THE PARTIES Gandhinagar, India, 17 - 22 February 2020 Agenda Item 27.1 PROPOSAL FOR THE INCLUSION OF THE ANTIPODEAN ALBATROSS (Diomedea antipodensis) ON APPENDIX I OF THE CONVENTION Summary: The Governments of New Zealand, Australia and Chile have submitted the attached proposal for the inclusion of the Antipodean albatross (Diomedea antipodensis) on Appendix I of CMS. The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CMS Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. UNEP/CMS/COP13/Doc. 27.1.7 PROPOSAL FOR INCLUSION OF THE ANTIPODEAN ALBATROSS (Diomedea antipodensis) ON APPENDIX I OF THE CONVENTION A. PROPOSAL Inclusion of Diomedea antipodensis on the Convention on the Conservation of Migratory Species of Wild Animals (CMS) Appendix I. The current CMS Appendix II listing will remain in place. Diomedea antipodensis is classified as Endangered (IUCN) as it is undergoing a very rapid decline in population size. B. PROPONENT: Governments of New Zealand, Australia and Chile. C. SUPPORTING STATEMENT 1. Taxonomy 1.1 Class: Aves 1.2 Order: Procellariiformes 1.3 Family: Diomedeidae (albatrosses) 1.4 Genus, species or subspecies, including author and year: Diomedea antipodensis (Robertson & Warham 1992), including two subspecies: Diomedea antipodensis antipodensis and Diomedea antipodensis gibsoni 1.5 Scientific synonyms: Diomedea exulans antipodensis Diomedea antipodensis was formerly included in the wandering albatross complex (Diomedea exulans) (e.g. -

Andrea Milković New Zealand and Its Tourism Potential
New Zealand and its Tourism Potential Milković, Andrea Undergraduate thesis / Završni rad 2017 Degree Grantor / Ustanova koja je dodijelila akademski / stručni stupanj: Polytechnic of Međimurje in Čakovec / Međimursko veleučilište u Čakovcu Permanent link / Trajna poveznica: https://urn.nsk.hr/urn:nbn:hr:110:471894 Rights / Prava: In copyright Download date / Datum preuzimanja: 2021-09-30 Repository / Repozitorij: Polytechnic of Međimurje in Čakovec Repository - Polytechnic of Međimurje Undergraduate and Graduate Theses Repository MEĐIMURSKO VELEUČILIŠTE U ČAKOVCU STRUČNI STUDIJ MENADŢMENT TURIZMA I SPORTA ANDREA MILKOVIĆ NEW ZEALAND AND ITS TOURISM POTENTIAL ZAVRŠNI RAD ČAKOVEC, 2016. POLYTECHNIC OF MEĐIMURJE IN ČAKOVEC PROFESSIONAL STUDY PROGRAME MANAGEMENT OF TOURISM AND SPORT ANDREA MILKOVIĆ NEW ZEALAND AND ITS TOURISM POTENTIAL FINAL PAPER Mentor: Marija Miščančuk, prof. ČAKOVEC, 2016 Zahvala: Veliku zahvalnost, u prvom redu, dugujem svojoj mentorici, prof. Mariji Miščančuk zbog savjetovanja, usmjeravanja i odvojenog vremena tijekom pisanja ovog završnog rada. Zahvaljujem se i ostalim djelatnicima na MeĎimurskom Veleučilištu u Čakovcu zbog kvalitetnog prenošenja znanja i pomoći tijekom studiranja. Veliko hvala Antoniju Kovačeviću i sestri Nikolini Milković na pomoći oko nabavljanja literature i tehničkoj podršci. Isto tako, zahvaljujem im se na ohrabrenju i moralnoj podršci za vrijeme pisanja rada, ali i tijekom cijelog studiranja. TakoĎer, hvala mojim prijateljima Goranu Haramasu, Martini Šestak, Petri Benotić, Petri Kozulić i Vinki Kugelman koji su bili uz mene i učinili ove studijske godine ljepšima. Hvala mojoj obitelji na podršci i strpljenju tokom studija. ABSTRACT Curiosity of people leads to traveling for pleasure to new places where they can visit and learn about historical buildings, natural beauty and anything that makes one country special, interesting and worth visiting. -

Table 1 Comprehensive International Points List
Table 1 Comprehensive International Points List FCC ITU-T Country Region Dialing FIPS Comments, including other 1 Code Plan Code names commonly used Abu Dhabi 5 971 TC include with United Arab Emirates Aden 5 967 YE include with Yemen Admiralty Islands 7 675 PP include with Papua New Guinea (Bismarck Arch'p'go.) Afars and Assas 1 253 DJ Report as 'Djibouti' Afghanistan 2 93 AF Ajman 5 971 TC include with United Arab Emirates Akrotiri Sovereign Base Area 9 44 AX include with United Kingdom Al Fujayrah 5 971 TC include with United Arab Emirates Aland 9 358 FI Report as 'Finland' Albania 4 355 AL Alderney 9 44 GK Guernsey (Channel Islands) Algeria 1 213 AG Almahrah 5 967 YE include with Yemen Andaman Islands 2 91 IN include with India Andorra 9 376 AN Anegada Islands 3 1 VI include with Virgin Islands, British Angola 1 244 AO Anguilla 3 1 AV Dependent territory of United Kingdom Antarctica 10 672 AY Includes Scott & Casey U.S. bases Antigua 3 1 AC Report as 'Antigua and Barbuda' Antigua and Barbuda 3 1 AC Antipodes Islands 7 64 NZ include with New Zealand Argentina 8 54 AR Armenia 4 374 AM Aruba 3 297 AA Part of the Netherlands realm Ascension Island 1 247 SH Ashmore and Cartier Islands 7 61 AT include with Australia Atafu Atoll 7 690 TL include with New Zealand (Tokelau) Auckland Islands 7 64 NZ include with New Zealand Australia 7 61 AS Australian External Territories 7 672 AS include with Australia Austria 9 43 AU Azerbaijan 4 994 AJ Azores 9 351 PO include with Portugal Bahamas, The 3 1 BF Bahrain 5 973 BA Balearic Islands 9 34 SP include -

ISO Country Codes
COUNTRY SHORT NAME DESCRIPTION CODE AD Andorra Principality of Andorra AE United Arab Emirates United Arab Emirates AF Afghanistan The Transitional Islamic State of Afghanistan AG Antigua and Barbuda Antigua and Barbuda (includes Redonda Island) AI Anguilla Anguilla AL Albania Republic of Albania AM Armenia Republic of Armenia Netherlands Antilles (includes Bonaire, Curacao, AN Netherlands Antilles Saba, St. Eustatius, and Southern St. Martin) AO Angola Republic of Angola (includes Cabinda) AQ Antarctica Territory south of 60 degrees south latitude AR Argentina Argentine Republic America Samoa (principal island Tutuila and AS American Samoa includes Swain's Island) AT Austria Republic of Austria Australia (includes Lord Howe Island, Macquarie Islands, Ashmore Islands and Cartier Island, and Coral Sea Islands are Australian external AU Australia territories) AW Aruba Aruba AX Aland Islands Aland Islands AZ Azerbaijan Republic of Azerbaijan BA Bosnia and Herzegovina Bosnia and Herzegovina BB Barbados Barbados BD Bangladesh People's Republic of Bangladesh BE Belgium Kingdom of Belgium BF Burkina Faso Burkina Faso BG Bulgaria Republic of Bulgaria BH Bahrain Kingdom of Bahrain BI Burundi Republic of Burundi BJ Benin Republic of Benin BL Saint Barthelemy Saint Barthelemy BM Bermuda Bermuda BN Brunei Darussalam Brunei Darussalam BO Bolivia Republic of Bolivia Federative Republic of Brazil (includes Fernando de Noronha Island, Martim Vaz Islands, and BR Brazil Trindade Island) BS Bahamas Commonwealth of the Bahamas BT Bhutan Kingdom of Bhutan -

New Zealand Subantarctic Islands Research Strategy
New Zealand Subantarctic Islands Research Strategy SOUTHLAND CONSERVANCY New Zealand Subantarctic Islands Research Strategy Carol West MAY 2005 Cover photo: Recording and conservation treatment of Butterfield Point fingerpost, Enderby Island, Auckland Islands Published by Department of Conservation PO Box 743 Invercargill, New Zealand. CONTENTS Foreword 5 1.0 Introduction 6 1.1 Setting 6 1.2 Legal status 8 1.3 Management 8 2.0 Purpose of this research strategy 11 2.1 Links to other strategies 12 2.2 Monitoring 12 2.3 Bibliographic database 13 3.0 Research evaluation and conditions 14 3.1 Research of benefit to management of the Subantarctic islands 14 3.2 Framework for evaluation of research proposals 15 3.2.1 Research criteria 15 3.2.2 Risk Assessment 15 3.2.3 Additional points to consider 16 3.2.4 Process for proposal evaluation 16 3.3 Obligations of researchers 17 4.0 Research themes 18 4.1 Theme 1 – Natural ecosystems 18 4.1.1 Key research topics 19 4.1.1.1 Ecosystem dynamics 19 4.1.1.2 Population ecology 20 4.1.1.3 Disease 20 4.1.1.4 Systematics 21 4.1.1.5 Biogeography 21 4.1.1.6 Physiology 21 4.1.1.7 Pedology 21 4.2 Theme 2 – Effects of introduced biota 22 4.2.1 Key research topics 22 4.2.1.1 Effects of introduced animals 22 4.2.1.2 Effects of introduced plants 23 4.2.1.3 Exotic biota as agents of disease transmission 23 4.2.1.4 Eradication of introduced biota 23 4.3 Theme 3 – Human impacts and social interaction 23 4.3.1 Key research topics 24 4.3.1.1 History and archaeology 24 4.3.1.2 Human interactions with wildlife 25 4.3.1.3 -

From Podes to Antipodes: New Dimensions in Mapping Global Time, Cost, and Distance
From Podes to Antipodes: New Dimensions in Mapping Global Time, Cost, and Distance by Matthew A. Zook* and Stanley D. Brunn** Department of Geography University of Kentucky, Lexington, KY 40506-0027 *[email protected] **[email protected] Paper prepared for Specialist Workshop on Globalization in the World-System: Mapping Change over Time University of California, Riverside February 7-8, 2004 DRAFT – DRAFT - DRAFT – DRAFT - DRAFT - DRAFT Do not distribute or reproduce DRAFT – DRAFT - DRAFT – DRAFT - DRAFT - DRAFT Table of Contents Thinking about Time, Space and Distance ................................................................................. 2 Representations of Time, Space and Distance............................................................................ 3 Thinking about and Representing Global Air Travel............................................................... 5 Description of the Data and its Generation................................................................................ 6 Data Analysis................................................................................................................................. 8 Summation of Physical, Cost and Time Distance....................................................................... 9 Explaining Cost and Time Distance ......................................................................................... 10 Representing Physical, Cost and Time Distance ...................................................................... 11 Conclusions and Future Directions .......................................................................................... -

Floating on a Malayan Breeze : Travels in Malaysia and Singapore Pdf, Epub, Ebook
FLOATING ON A MALAYAN BREEZE : TRAVELS IN MALAYSIA AND SINGAPORE PDF, EPUB, EBOOK Sudhir Thomas Vadaketh | 324 pages | 18 Dec 2012 | Hong Kong University Press | 9789888139316 | English | Hong Kong, Hong Kong Floating on a Malayan Breeze : Travels in Malaysia and Singapore PDF Book This is probably because of my race and character. A must read for anyone now working in this part of the world for a good appreciation of the peculiarities and achievements of the two countries. Bucking this economic pragmatism is ridiculously difficult. Index pp. The only North Indian group that is widely accepted and instantly recognisable—because of its critical mass and distinctive dress—is the Sikh Punjabis. Forty-five years ago Singapore separated from Malaysia. Terence Loading In Floating , there is also a chapter dedicated to discussing the effects Mainland Chinese workers are having on the Singaporean society and labour force. She is a first-generation Singaporean Marwari, people who hail from Rajasthan in North-west India, about as far as you can get geographically and culturally from Kerala. Malaysia has given preference to the majority Malay Muslims — the bumiputera , or sons of the soil. Bounced around a bit too much for me as well, but it was my first good entre into that part of the world. He writes that "since , Malaysia and Singapore have tried hard to create distinct nation states. Immigration, meanwhile, is a complex issue that deserves its own piece. Since then, a combination of forces--including policy missteps by the ruling parties, the emergence of more credible opposition candidates, and the widening of political space through the internet--has blown the lid off our hitherto politically apathetic countries" p. -

Antipodes Global Fund
Antipodes Asia Fund ARSN 096 451 393 APIR IOF0203AU MONTHLY REPORT | 30 April 2021 Commentary Global equity strength continued in April as a further supportive US fiscal Key contributors included: backdrop boosted sentiment (+2.9). Investors exhibited a preference for • Consumer Defensive cluster, notably sportswear name Li Ning amidst momentum and growth over low multiple - or value - stocks with an ongoing consumer backlash against foreign competitors boycotting Communication Services and Information Technology outperforming. Cyclical the use of cotton from the Xinjiang region, and Wuliangye which reported sectors such as Materials, Financials and Consumer Discretionary were also strong revenues amidst supply constraints from a key competitor. strong while Energy was the major laggard. • Industrials, notably LG Chem on strong results across its chemicals, electronics and batteries businesses, and POSCO resulting from US equities (+3.9%) outperformed as US technology giants resumed strength in the price of steel. leadership, helped by stabilising yields. President Biden's speech to congress • Hardware cluster, notably MediaTek which reported strong first quarter signalled further fiscal spending plans funded by new tax proposals. Europe growth in revenues, primarily in 5G handset chip sales, while also lifting (+3.1%) marginally outperformed as the trajectory for COVID-19 vaccinations near term guidance, and TSMC which also lifted 2021 revenue guidance. improved. Alchip was a notable exception following a US decision to add Chinese supercomputing entities with links to the Chinese military, which included Asia (-0.4%) underperformed as Japan (-2.9%) weighed due to rising COVID- Alchip clients, to its economic blacklist. 19 infections, a slow vaccine rollout, and a new state of emergency. -

Northern Giant Petrel Macronectes Halli Breeding Population Survey, Auckland Islands
Northern giant petrel Macronectes halli breeding population survey, Auckland Islands December 2015 – February 2016 Graham C. Parker, Chris G. Muller and Kalinka Rexer‐Huber Department of Conservation, Conservation Services Programme, Contract 4655‐4 1 Northern giant petrel Macronectes halli breeding population survey, Auckland Islands December 2015– February 2016 Department of Conservation, Conservation Services Programme, Contract 4655‐4 Graham C. Parker1*, Chris G. Muller3 & Kalinka Rexer‐Huber1,2 1 Parker Conservation, 126 Maryhill Tce, Dunedin New Zealand 2 University of Otago, PO Box 56, Dunedin New Zealand 3Massey University Manawatu, Private Bag 11 222, Palmerston North 4442, New Zealand *Corresponding author: [email protected] Please cite as: Parker, G.C., Muller, C.G., Rexer‐Huber, K. 2016. Northern giant petrel Macronectes halli breeding population survey, Auckland Islands, December 2015 – February 2016. Report to the Conservation Services Programme, Department of Conservation. Parker Conservation, Dunedin 2 Executive summary Northern giant petrels Macronectes halli are a large, southern hemisphere fulmarine petrel that face conservation threats both in the terrestrial and marine environment. Introduced mammalian predators at breeding sites cause nesting failures and in some instances may also depredate adults. In the marine environment Northern giant petrels are threatened by capture in longline and trawl fisheries, oil pollution, shooting by fishers for bait stealing and the effects of climate change. The contemporary size and the population trends of Northern giant petrels on New Zealand islands are not known. Records of their numbers in the Auckland Islands are based solely on anecdotal evidence, and the most recent summary dates to the 1980s. We estimated the size of the Northern giant petrel breeding population and describe their spatial distribution in the Auckland Islands. -

Managementframeworksf
Papers and Proceedings ofthe Royal Society of Tasmania, Volume 141(1), 2007 29 MANAGEMENTFRAMEWORKSFOR THE NEWZEALAND SUB-ANTARCTIC ISLANDS by A. D. Roberts Roberts, A.D. 2007 (23:xi): Management frameworks for the New Zealand sub-Antarctic islands. Papers and Proceedings of the Royal Society of Tasmania 141(1): 29-32. https://doi.org/10.26749/rstpp.141.1.29 ISSN 0080-4703. Department of Conservation, PO Box 743, Invercargill, New Zealand. Email: [email protected] Thefive island groups known as the New Zealand sub-Antarctic islands - Antipodes Islands, Auckland Islands, Bounty Islands, Campbell Island and Snares Islands - are perhaps better regarded as cool temperate although they share many features and many management issues with islands south of the Antarctic Convergence. The Snares and Bounty islands have granitic substrates; the Antipodes, Auckland and Campbell islands are of volcanic origin. Marine mammals and seabirds are important components of the fauna. Terrestrial Hora and inver tebrate fauna show high levels of endemism. Several species of alien vertebrates have been successfully eradicated. The islands are managed by the New Zealand Department of Conservation as National Nature Reserves, and together are recognised as a World Heritage Site. Key Words: sub-Antarctic islands, cool temperate, Antipodes Islands, Auckland Islands, Bounty Islands, Campbell Island, Snares Islands, World Heritage Site, alien species removal. TheNew Zealand sub-Antarctic islands are located south of There is evidence of Polynesian v1s1tation in the pre New Zealand in the Pacific sector of the Southern Ocean. European period (Peat 2003). European discovery was in Although these islands are not truly sub-Antarctic but more 1791. -
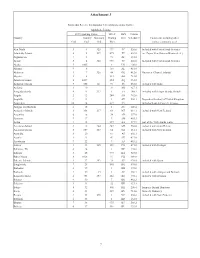
Attachment 3
Attachment 3 Points that Receive International Telecommunications Traffic: Alphabetic Listing FCC reporting Codes ITU-T FIPS Census Country Country Summary Dialing 10-3 Schedule C Comments, including other Code Code Code Plan names commonly used Abu Dhabi 3 1 325 971 TC 520.0 Included with United Arab Emirates Admiralty Islands 8 2 237 675 PP 604.0 inc. Papua New Guinea (Bismarck A.) Afghanistan 7 3 93 AF 531.0 Ajman 3 4 325 971 TC 520.0 Included with United Arab Emirates Alaska 5 1005 1 US 100.0 Albania 9 6 355 AL 481.0 Alderney 1 7 326 44 GK 412.0 Guernsey (Channel Islands) Algeria 2 8 213 AG 721.0 American Samoa 8 1009 684 AQ 951.0 Andaman Islands 7 355 141 91 IN 533.0 included with India Andorra 1 10 33 AN 427.1 Anegada Islands 4 11 337 1 VI 248.2 included with Virgin Islands, British Angola 2 12 244 AO 762.0 Anguilla 4 13 1 AV 248.1 Dependent territory of United Kingdom Antarctica 10 14 672 AY Includes Scott & Casey U.S. bases Antigua and Barbuda 4 15 1 AC 248.4 Antipodes Islands 8 356 217 64 NZ 614.1 included with New Zealand Argentina 6 16 54 AR 357.0 Armenia 9 17 7 AM 463.1 Aruba 4 18 297 AA 277.9 part of the Netherlands realm Ascension Island 2 19 264 247 SH 758.0 included with Saint Helena Auckland Islands 8 357 217 64 NZ 614.1 included with New Zealand Australia 8 20 61 AS 602.1 Austria 1 21 43 AU 433.0 Azerbaijan 9 22 7 AJ 463.2 Azores 1 23 245 351 PO 471.0 included with Portugal Bahamas, The 4 24 1 BF 236.0 Bahrain 3 25 973 BA 525.0 Baker Island 8 1026 11 FQ 980.0 Balearic Islands 1 27 293 34 SP 470.0 included with Spain -

Download Download
Number 63, Spring 2009 cartographic perspectives 69 its chapters, the text is to be highly recommended as generations, it was gradually supplemented by new encouraging further work in this realm. Because of this knowledge, which led to the need to distinguish book, and other publications by Knowles, Hillier, Bol, ancient from modern knowledge. For Hiatt, there is Gregory, and others, I look forward to future publica- a clear and consistent, if not always smooth, line of tions in the use of GIS for History. thought extending from the ancient period through the early modern. It is characterized by ancient writings being retold and supplemented, not simply to preserve the originals, but to give them prolonged credibility by Terra Incognita: Mapping the Antipodes before 1600 making them appear to foretell subsequent discover- By Alfred Hiatt ies. University of Chicago Press, 2008 The third theme explores periodization, and Hiatt’s 298 pages, 8 color plates, 47 grayscale figures conviction that conceptions of the world do not easily Hardbound (ISBN 13: 978-0-226-33303-8) fit standard period delimiters. He observes that, while change did occur, “[W]hy that change occurred will Reviewed by: Jonathan F. Lewis not be enlightening if it falls back on banalities about Benedictine University inherently “antique,” “medieval,” or “modern” ways of viewing the world” ( 9). For Hiatt, the medieval pe- Terra Incognita examines and explains the initial ap- riod witnessed not the end of a view of the world in- pearance and subsequent evolution of European per- formed by ancient writers, but rather a dialogue with spectives on remote, unvisited portions of the globe.