Crustacea: Gammaridae) from Krubera Cave (Western Transcaucasia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reviews of the Genera Schaefferia Absolon, 1900, Deuteraphorura
TAR Terrestrial Arthropod Reviews 5 (2012) 35–85 brill.nl/tar Reviews of the genera Schaefferia Absolon, 1900, Deuteraphorura Absolon, 1901, Plutomurus Yosii, 1956 and the Anurida Laboulbène, 1865 species group without eyes, with the description of four new species of cave springtails (Collembola) from Krubera-Voronya cave, Arabika Massif, Abkhazia Rafael Jordana1, Enrique Baquero1*, Sofía Reboleira2 and Alberto Sendra3 1Department of Zoology and Ecology, University of Navarra, 31080 Pamplona, Spain e-mails: [email protected]; [email protected] *Corresponding author. 2Department of Biology, Universidade de Aveiro and CESAM Campus Universitário de Santiago, 3810-193 Aveiro, Portugal e-mail: [email protected] 3Museu Valencià d’Història Natural (Fundación Entomológica Torres Sala) Paseo de la Pechina 15. 46008 Valencia, Spain e-mail: [email protected] Received on November 4, 2011. Accepted on November 21, 2011 Summary Krubera-Voronya cave and other deep systems in Arabika Massif are being explored during many speleological expeditions. A recent Ibero-Russian exploration expedition (summer of 2010) took place in this cave with the aim of providing a study of the biocenosis of the deepest known cave in the world. Four new species of Collembola were found at different depths: Schaefferia profundissima n. sp., Anurida stereoodorata n. sp., Deuteraphorura kruberaensis n. sp., and Plutomurus ortobalaganensis n. sp., the last one at -1980 m deep. The identification and description of the new species have required the careful study of all congeneric species, implying a revision of each genus. As a result of this work tables and keys to all significant characters for each species are presented. -

By Jocelyn Cranefield
Expository Text by Jocelyn Cranefield PAIRED READ Why Bat Flies at Night STRATEGIES & SKILLS Comprehension Content Standards Strategy: Ask and Answer Science Questions Life Science Skill: Cause and Effect Vocabulary adaptations, agile, caches, dormant, forage, frigid, hibernate, insulate Word Count: 1,434** Photography Credit: MICHAEL NICHOLS/National Geographic Creative **The total word count is based on words in the running text and headings only. Numerals and words in captions, labels, diagrams, charts, and sidebars are not included. mheducation.com/prek-12 Copyright © 2014 McGraw-Hill Education All rights reserved. No part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written consent of McGraw-Hill Education, including, but not limited to, network storage or transmission, or broadcast for distance learning. Send all inquiries to: McGraw-Hill Education Two Penn Plaza New York, New York 10121 ISBN: 978-0-02-119233-5 MHID: 0-02-119233-2 Printed in the United States of America. 11 12 13 14 15 QVS 22 21 20 19 18 E Genre Expository Text Essential Question How are living things adapted to their environment? by Jocelyn Cranefield Introduction ................................ 2 Chapter 1 From the Entrance to the Twilight Zone ..... 4 Chapter 2 Dark and Surprising Places ................. 10 Conclusion.................................. 16 Respond to Reading ........................ 18 PAIRED READ Why Bat Flies at Night ........... 19 Glossary ................................... 22 Index ...................................... 23 Focus on Science ................. 24 Introduction Caves are found all over the world. They often go a long way in from the entrance and go deep below Earth’s surface. -

TABLE of CONTENTS Vol. 2
TABLE OF CONTENTS Vol. 2 O-126 Classification of karst features in Mount Lebanon F. Nader • American University of Beirut / Speleo-Club du Liban, Beirut, Lebanon..........................................................................375 O-127 Cave Ulica and the denudation of the karst surface - case study from Kras, SW Slovenia A. Mihevc • Karst research Institute ZRC SAZU, Postojna, Slovenia.................................................................................................375 O-128 The underground legend of Carbon Dioxide heaviness G. Badino • Dip. Fisica Generale, Universita di Torino......................................................................................................................375 O-129 Calibrated Holocene Paleotemperature Record for North America from Stable Isotopic Analyses of Speleothems and their Fluid Inclusions P.A. Beddows, R. Zhang, D.C. Ford, H.P. Schwarcz • School of Geography & Geology, McMaster University, Hamilton, Ontario, Canada...................................................................................................................................................................................................379 O-130 Origin of the Climatic Cycles from Orbital to Sub-Annual: Speleothem data Y.Y. Shopov, D. Stoykova, L.T. Tsankov, D.C. Ford, C.J. Yonge • University Center for Space Research and Technologies, University of Sofia,Bulgaria..................................................................................................................................................................379 -

(Krubera) Cave
Compass & Tape, Volume 17, Number 3, Issue 59 HYDROLEVELING OF VERY DEEP CAVES, WITH AN EXAMPLE FROM VORONJA (KRUBERA) CAVE BY ALEXANDER DEGTJAREV, EUGENE SNETKOV, AND ALEXEY GURJANOV REPRINTED FROM AMCS ACTIVITIES NEWSLETTER, NO. 29 This interesting article on a technique in the beginning of the 1970s. The de- Cave in the Bzybsky Ridge. The length for measuring the depth of a deep cave vice was a vinyl-coated fabric tube 30 of a river passage from the resurgence with high accuracy appeared in Svet to 50 meters in length with a manom- sump at the entrance, called a gryphon (The Light), magazine of the Ukrainian eter with fine scale divisions at one end. by Russian cavers, to the terminal sump Speleological Association, number 29, Such manometers were 25 centimeters is about 1.5 kilometers, and the eleva- 2006. It has been revised and rear- or more in diameter. They were incon- tion difference is only 20 meters. Gena ranged by the editor based on a trans- venient and often broken. The water Somokhin of Ukraine did a hydro- lation for AMCS from Russian by bottle that was used to keep the tube leveling in V. Pantjukhina Cave in the Tatyana Nemchenko, who also kindly filled overturned, and bubbles appeared same area, and the depth of the cave provided some clarifications by e-mail. in the tube. Nevertheless, the method changed from 1508 to 1488 meters. Authors and translator are Moscovites. was used and considered the most ac- New interest in the method appeared curate method of determining depths at after discovery of the deepest cave, n expedition to Voronja Cave was the time. -

Alexander Klimchouk Oral History Interview with Dr. Bogdan Onac, January 23, 2007 A
University of South Florida Scholar Commons Environmental Sustainability Oral Histories Environmental Sustainability 1-23-2007 Alexander Klimchouk oral history interview with Dr. Bogdan Onac, January 23, 2007 A. B. Klimchuk (Interviewee) Bogdan P. Onac (Interviewer) University of South Florida, [email protected] Follow this and additional works at: http://scholarcommons.usf.edu/tles_oh Part of the Sustainability Commons Scholar Commons Citation Klimchuk, A. B. (Interviewee) and Onac, Bogdan P. (Interviewer), "Alexander Klimchouk oral history interview with Dr. Bogdan Onac, January 23, 2007" (2007). Environmental Sustainability Oral Histories. Paper 1. http://scholarcommons.usf.edu/tles_oh/1 This Oral History is brought to you for free and open access by the Environmental Sustainability at Scholar Commons. It has been accepted for inclusion in Environmental Sustainability Oral Histories by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. COPYRIGHT NOTICE This Oral History is copyrighted by the University of South Florida Libraries Oral History Program on behalf of the Board of Trustees of the University of South Florida. Copyright, 2011, University of South Florida. All rights, reserved. This oral history may be used for research, instruction, and private study under the provisions of the Fair Use. Fair Use is a provision of the United States Copyright Law (United States Code, Title 17, section 107), which allows limited use of copyrighted materials under certain conditions. Fair Use limits the amount of material that may be used. For all other permissions and requests, contact the UNIVERSITY OF SOUTH FLORIDA LIBRARIES ORAL HISTORY PROGRAM at the University of South Florida, 4202 E. -

New Species of Beetle Discovered in the World's Deepest Cave 1 July 2014
New species of beetle discovered in the world's deepest cave 1 July 2014 "The new species of cave beetle is called Duvalius abyssimus. We only have two specimens, a male and a female. Although they were captured in the world's deepest cave, they were not found at the deepest point," Ortuño, who has dedicated the last 10 years to studying subterranean fauna, declared to SINC. The Duvalius genus is a successful colonizer of the Earth's depths. The majority of species have a hypogean lifestyle and live in caves or the superficial underground compartment. "The new species' characteristics indicate that it is moderately adapted to life underground. Proof of this is that they still have eyes, which are absent in A drawing of Duvalius abyssimus. Credit: José Antonio the highly specialised cave species," added the Peñas expert. The Arabika massif region in Abkhazia, where this cave is found, is biogeographically a very The unusual habitat of the Krubera cave in the interesting area. Altitudes fluctuate between 1,900 Western Caucasus remains a mystery. and 2,500 metres and the cave is composed of Researchers from two Spanish universities have lower and upper Jurassic-Cretaceous limestone. discovered a new species of beetle in the depths of this cave. Its large area has provided endless subterranean refuges for fauna. In fact, various genera of Cave beetles are one of the most iconic species endemic cave beetles live in the Western found in subterranean habitats. They were Caucasus. "Its location is strategic, since there are historically the first living organisms described by fauna of European, Asian and also endemic origin science that are adapted to the conditions of in the zone," the scientist underlined. -

World Heritage Caves and Karst: a Thematic Study
World Heritage Convention IUCN World Heritage Studies 2008 Number Two World Heritage Caves and Karst A Thematic Study IUCN Programme on Protected Areas A global review of karst World Heritage properties: present situation, future prospects and management requirements . IUCN, International Union for Conservation of Nature Founded in 1948, IUCN brings together States, government agencies and a diverse range of non-governmental organizations in a unique worldwide partnership: over 1000 members in all, spread across some 140 countries. As a Union, IUCN seeks to influence, encourage and assist societies throughout the world to conserve the integrity and diversity of nature and to ensure that any use of natural resources is equitable and ecologically sustainable. A central Secretariat coordinates the IUCN Programme and serves the Union membership, representing their views on the world stage and providing them with the strategies, services, scientific knowledge and technical support they need to achieve their goals. Through its six Commissions, IUCN draws together over 10,000 expert volunteers in project teams and action groups, focusing in particular on species and biodiversity conservation and the management of habitats and natural resources. The Union has helped many countries to prepare National Conservation Strategies, and demonstrates the application of its knowledge through the field projects it supervises. Operations are increasingly decentralized and are carried forward by an expanding network of regional and country offices, located principally in developing countries. IUCN builds on the strengths of its members, networks and partners to enhance their capacity and to support global alliances to safeguard natural resources at local, regional and global levels. -

Speleological Investigation of the Largest Limestone Massif in Georgia (Caucasus)
Open Journal of Geology, 2017, 7, 1530-1537 http://www.scirp.org/journal/ojg ISSN Online: 2161-7589 ISSN Print: 2161-7570 Speleological Investigation of the Largest Limestone Massif in Georgia (Caucasus) Lasha Asanidze1, Zaza Lezhava1, Nino Chikhradze1,2 1Vakhushti Bagrationi Institute of Geography, Ivane Javakhishvili Tbilisi State University, Tbilisi, Georgia 2School of Natural Sciences and Engineering, Ilia State University, Tbilisi, Georgia How to cite this paper: Asanidze, L., Lez- Abstract hava, Z. and Chikhradze, N. (2017) Speleo- logical Investigation of the Largest Limes- Georgia is home to multiple, widespread limestone massifs with well-devel- tone Massif in Georgia (Caucasus). Open oped karst areas and their associated landscape features found throughout the Journal of Geology, 7, 1530-1537. country. Due to geological, geomorphological, and speleological characteris- https://doi.org/10.4236/ojg.2017.710102 tics of the limestone massifs in Georgia, there are developments in classical Received: September 26, 2017 karst processes and landforms, which contain very impressive karst features, Accepted: October 23, 2017 such as dolines, caves, calcite depositions and others. For example, in Georgia, Published: October 26, 2017 the world’s deepest caves are found, such as: Krubera-2197 m; Sarma-1830 m; Pantyukhina-1508; Ilyukhina-1275 m; Kuibyshev-1110 m, and others. Of Copyright © 2017 by authors and these, Krubera Cave is currently the deepest in the world. The goal of this Scientific Research Publishing Inc. This work is licensed under the Creative work is to present speleological investigation of Muradi Cave, which is devel- Commons Attribution International oped in Racha limestone massif. Muradi Cave is unique as the fact that it con- License (CC BY 4.0). -

A. Packet 1.Pdf
Florida Spring Tournament 2019 Packet 1 Edited by: Taylor Harvey, Tracy Mirkin, Jonathen Settle, and Alex Shaw Written by: Jason Freng, David Gunderman, Paul Hansel, Taylor Harvey, Jacob Hujsa, Bradley Kirksey, Leo Law, Tracy Mirkin, Matt Mitchell, Jacob Murphy, Jonathen Settle, Alex Shaw, and Chandler West TOSSUPS: 1. A painting of a figure of this name is reproduced in the bottom right of The Tribuna of the Uffizi by Johan Zoffany. A nymph uses a mirror to check votive offerings to a statue of this figure in the top right of painting whose bottom left depicts dozens of putti. Giorgione depicted a nude version of this figure reclining on a hillside in a painting alternatively called Dresden or (*) Sleeping [this figure]. A girl kneels in prayer and a small dog lies sleeping in a Titian painting titled for this figure. Zephyrus blows flowers toward this figure as she covers her genitals with her long hair in a depiction of her standing atop a shell. For 10 points, name this Roman goddess whose birth was painted by Sandro Botticelli. ANSWER: Venus [accept Worship of Venus, Sleeping Venus, Venus of Urbino, or Birth of Venus] <TH, Painting> 2. An effect driven by dipolar coupling between these structures depends on the cross-relaxation rate and is modeled with the Solomon equations. These structures are stationary in the Born-Oppenheimer approximation, leading to a "clamped" Schrödinger equation. Interactions between the spin of these structures mediate J coupling, and the difference in the precession frequency of these structures' spin is reported as chemical (*) shift. The discovery of this structure was compared to firing a “15-inch shell at a piece of tissue paper and it [coming] back and [hitting] you”; that quote is by Ernest Rutherford, who discovered these structures in his gold foil experiment. -

Notable Caves of the World
NOTABLE CAVES OF THE WORLD Name Location Interesting Facts • ceiling paintings discovered in 1879 by an eight-year- Spain Altamira old girl 43.377 °N, 4.123 °W • noted for its many cave paintings New Mexico • altitude and insulating effects of lava cave walls Bandera Volcano Ice Cave 34.991 °N, 108.083 °W maintain blue-green ice formations year-round Italy • sea cave that seems to be lit from underwater giving Blue Grotto 40.561 °N, 14.206 °E it its beautiful blue color • largest cavern in the United States (One chamber, the New Mexico Carlsbad Caverns Big Room, could hold an aircraft carrier!) 32.177 °N, 104.441 °W • contains Indian wall paintings Austria Eisriesenwelt • world’s largest ice cave—42 km in length 47.503 °N, 13.189 °E • cave paintings discovered in 1940 France • famous Hall of Bulls Grotto de Lascaux 45.054 °N, 1.168 °E • original caves closed due to deterioration caused by visitors Abkhazia, Georgia Krubera Cave • deepest known cave in the world at 2197 m 43.409 °N, 40.361 °E California Lava Beds National Monument • world’s largest collection of volcanic caves 41.707 °N, 121.514 °W • many spectacular formations Virginia Luray Caverns • a stalacpipe organ that creates musical notes by 38.664 °N, 78.484 °W striking hollow stalactites Kentucky • most extensive known cave in the world, with Mammoth Cave 37.186 °N, 86.101 °W 652 km of passages and rooms China Miao Room of the Gebihe Cave • large cave chamber—852 m × 191 m × 66 m 25.677 °N, 106.265 °E Mexico Ox Bel Ha • most extensive known underwater cave—270 km 20.160 °N, 87.488 °W • desert caves near the Dead Sea where ancient scrolls Israel Qumran Caves were found, including parts of all Old Testament 31.741 °N, 35.459 °E books except Esther Borneo • world’s largest known cave chamber by area— Sarawak Chamber 4.024 °N, 114.824 °E 600 m × 435 m × 115 m New Zealand • Glowworms cling to the ceiling, giving the Waitomo Caves 38.261 °S, 175.103 °E appearance of stars in a night sky. -
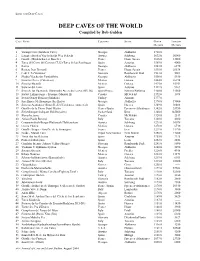
Long and Deep Caves of the World
LONG AND DEEP CAVES DEEP CAVES OF THE WORLD Compiled by Bob Gulden CAVE NAME COUNTRY STATE DEPTH LENGTH METERS METERS 1 Voronja Cave (Krubera Cave) Georgia Abkhazia 1710.0 ----- 2 Lamprechtsofen Vogelschacht Weg Schacht Austria Salzburg 1632.0 50000 3 Gouffre Mirolda/Lucien Bouclier France Haute Savoie 1626.0 13000 4 Torca del Cerro del Cuevon (T.33)-Torca de las Saxifragas Spain Asturias 1589.0 4000 5 Sarma Georgia Abkhazia1543.0 6370 6 Reseau Jean Bernard France Haute Savoie 1535.0 20536 7 Cehi 2 “la Vendetta” Slovenia Rombonski Podi 1533.0 5061 8 Shakta Vjacheslav Pantjukhina Georgia Abkhazia 1508.0 5530 9 Sistema Cheve (Cuicateco) Mexico Oaxaca 1484.0 26194 10 Sistema Huautla Mexico Oaxaca 1475.0 55953 11 Sistema del Trave Spain Asturias 1441.0 9167 12 Sima de las Puertas de Illaminako Ateeneko Leizea (BU.56) Spain/France Navarra/Nafarroa 1408.0 14500 13 Sustav Lukina jama - Trojama (Manual II) Croatia Mt.Velebit 1392.0 1078 14 Evren Gunay Dudeni (Sinkhole) Turkey Anamur 1377.0 ----- 15 Sniezhnaja-Mezhonnogo (Snezhaya) Georgia Abkhazia 1370.0 19000 16 Sistema Aranonera (Sima S1-S2)(Tendenera connected) Spain Huesca 1349.0 36468 17 Gouffre de la Pierre Saint Martin France/Spain Pyrenees-Atlantiques 1342.0 53950 18 Siebenhengste-hohgant Hohlensystem Switzerland Bern 1340.0 145000 19 Slovacka jama Croatia Mt.Velebit 1320.0 2519 20 Abisso Paolo Roversi Italy Toscana 1300.0 4000 21 Cosanostraloch-Berger-Platteneck Hohlesystem Austria Salzburg 1291.0 30076 22 Cueva Charco Mexico Oaxaca 1278.0 6710 23 Gouffre Berger - Gouffre de la Fromagere France Isere 1271.0 31790 24 Neide - Muruk Cave Papua New Guinea New Britain 1258.0 17000 25 Torca dos los Rebecos Spain Asturias 1255.0 2228 26 Pozo del Madejuno Spain Leon 1252.0 2852 27 Crnelsko brezno (Abisso Veliko Sbrego) Slovenia Rombonaki Podi 1241.0 8090 28 Vladimir V. -

Zoology in the Middle East, 2018
Zoology in the Middle East ISSN: 0939-7140 (Print) 2326-2680 (Online) Journal homepage: http://www.tandfonline.com/loi/tzme20 The hypogean invertebrate fauna of Georgia (Caucasus) Shalva Barjadze, Zezva Asanidze, Alexander Gavashelishvili & Felipe N. Soto- Adames To cite this article: Shalva Barjadze, Zezva Asanidze, Alexander Gavashelishvili & Felipe N. Soto- Adames (2018): The hypogean invertebrate fauna of Georgia (Caucasus), Zoology in the Middle East, DOI: 10.1080/09397140.2018.1549789 To link to this article: https://doi.org/10.1080/09397140.2018.1549789 View supplementary material Published online: 11 Dec 2018. Submit your article to this journal View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tzme20 Zoology in the Middle East, 2018 http://dx.doi.org/10.1080/09397140.2018.1549789 The hypogean invertebrate fauna of Georgia (Caucasus) Shalva Barjadzea, Zezva Asanidzeb,*, Alexander Gavashelishvilib and Felipe N. Soto-Adamesc aInstitute of Zoology, Ilia State University, Tbilisi, Georgia; b,*Institute of Ecology, Ilia State University, Tbilisi, Georgia; cDivision of Plant Industry, Florida State Collection of Arthropods, Florida Department of Agriculture and Consumer Services, Gainesville, USA (Received 29 June 2018; accepted 13 November 2018) Information about the cave invertebrates of Georgia, Caucasus, is summarised, resulting in 43 troglo- and 43 stygobiont taxa reported from 64 caves. Species distribution analyses were conducted for 61 caves harbouring 58 invertebrate taxa, with the majority of caves (39) located in Apkhazeti (north-western Georgia). In 22 caves from central-west Georgia (Samegrelo, Imereti and Racha-Lechkhumi regions of west Georgia) 31 taxa are reported.