Cobalt Production in Pennsylvania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Pennsylvania State University Center for Critical Minerals Final
The Pennsylvania State University Center for Critical Minerals Final Report on Cobalt Production in Pennsylvania: Context and Opportunities PREPARED FOR LEONARDO TECHNOLOGIES LLC., PREPARED BY Peter L. Rozelle Feifei Shi Mohammad Rezaee Sarma V. Pisupati Disclaimer This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. ii Executive Summary Cobalt is a critical mineral as defined by the U.S. Department of the Interior in response to Executive Order 13817 (2017). Cobalt metal is used in superalloys required in the hot gas path of stationary gas turbines and jet engines, as well as in samarium cobalt magnets, the domestic production capability of which was found to be essential to the National Defense under Presidential Determination 2019-20. Cobalt compounds are used in lithium-ion batteries, as are manganese compounds. The Defense Industrial Base Report, in response to Executive Order 13806 (2017), expressed concerns regarding the U.S. -

Overview of the Nickel Market in Latin America and the Caribbean
INSG SECRETARIAT BRIEFING PAPER April 2021 – No.35 Overview of the Nickel market in Latin America and the Caribbean Ricardo Ferreira, Director of Market Research and Statistics Francisco Pinto, Manager of Statistical Analysis 1. Introduction At the suggestion of the Group’s Brazilian delegation, it was agreed by members that a report, based on INSG Insight No. 26, published in November 2015 entitled “An overview of the nickel industry in Latin America”, be prepared by the secretariat. This would include an update of the operations that resumed production (e.g. Falcondo in the Dominican Republic), and also discuss how the emerging battery market might influence nickel usage in the region as well as the possibility of nickel scrap usage. This detailed and comprehensive Insight report, the 35th in the series of INSG Insight briefing reports, provides members with the results of that research work. In Latin America and the Caribbean, the nickel producing countries are Brazil, Colombia, Cuba, Dominican Republic, Guatemala and Venezuela. The Dominican Republic stopped nickel mining and smelting in October 2013 but resumed production at the beginning of 2016. Venezuela has not produced since mid-2015. All of these countries mine nickel and process it further to produce intermediate or primary nickel – mainly ferronickel. Most of the mined ore is processed within each country, and then exported to overseas markets, but Brazil and Guatemala also export nickel ore. In terms of usage only Brazil and Mexico are relevant regarding the global nickel market. The first section of this report briefly describes existing nickel deposits in the region. -

Rebel Attacks on Nickel Ore Mines in the Surigao Del Norte Region
Mindanao, Philippines – rebel attacks on nickel ore mines in the Surigao del Norte region The attacks On 3 October 2011, three mining firms located in and around Claver town in the Surigao del Norte region of Mindanao, Philippines were attacked by the rebel group known as the New People’s Army (NPA). It was reported that the attacks were prompted by a refusal of the mining firms to pay a "revolutionary tax" demanded by the NPA. The affected mining firms are the Taganito Mining Corporation (TMC), Taganito HPAL Nickel Corporation and Platinum Group Metals Corporation (PGMC)1. According to news reports and Gard’s local correspondent in the Philippines, some 300 NPA rebels wearing police/military uniforms staged coordinated strikes on the three mining facilities. The rebels attacked TMC’s compound first, burning heavy equipment, service vehicles, barges and buildings. Other rebel groups simultaneously attacked the nearby PGCM and Taganito HPAL compounds. Unconfirmed reports indicate that, unfortunately, casualties were also experienced during the attacks. All foreign vessels anchored near Taganito at the time of the attacks left the anchorage safely. The current situation The Philippine Government has stated that the situation is now contained and has deployed additional troops in the area to improve security and pursue the NPA rebels. However, according to Gard’s local correspondent in the Philippines, threats of further attacks made by the NPA in a press release are believed by many to be real. According to the latest press releases (Reuters on 5 October), Nickel Asia Corporation has resumed operations at its biggest nickel mine TMC and expects to ship ore in the next three weeks. -
Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254)
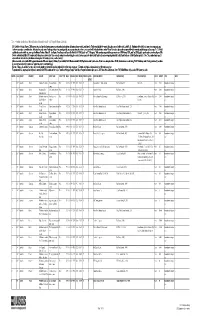
Table1.—Attribute data for the map "Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254). [The United States Geological Survey (USGS) surveys international mineral industries to generate statistics on the global production, distribution, and resources of industrial minerals. This directory highlights the economically significant mineral facilities of Asia and the Pacific. Distribution of these facilities is shown on the accompanying map. Each record represents one commodity and one facility type for a single location. Facility types include mines, oil and gas fields, and processing plants such as refineries, smelters, and mills. Facility identification numbers (“Position”) are ordered alphabetically by country, followed by commodity, and then by capacity (descending). The “Year” field establishes the year for which the data were reported in Minerals Yearbook, Volume III – Area Reports: Mineral Industries of Asia and the Pacific. In the “DMS Latitiude” and “DMS Longitude” fields, coordinates are provided in degree-minute-second (DMS) format; “DD Latitude” and “DD Longitude” provide coordinates in decimal degrees (DD). Data were converted from DMS to DD. Coordinates reflect the most precise data available. Where necessary, coordinates are estimated using the nearest city or other administrative district.“Status” indicates the most recent operating status of the facility. Closed facilities are excluded from this report. In the “Notes” field, combined annual capacity represents the total of more facilities, plus additional -

DOGAMI MP-20, Investigations of Nickel in Oregon
0 C\1 a: w a.. <( a.. en ::::> 0 w z <( __j __j w () en � INVESTIGATIONS OF NICKEL IN OREGON STATE OF OREGON DEPARTMENT OF GEOL.OGY AND MINERAL. IN OUSTRIES DONAL.D .A HUL.L. STATE GEOLOGIST 1978 STATE OF OREGON DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES 1069 State Office Building, Portland, Oregon 97201 MISCELLANEOUS PAPER 20 INVESTIGATIONS OF NICKEL IN OREGON Len Ramp, Resident Geologist Grants Pass Field Office Oregon Department of Geology and Mineral Industries Conducted in conformance with ORS 516.030 . •. 5 1978 GOVERNING BOARD Leeanne MacColl, Chairperson, Portland Talent Robert W. Doty STATE GEOLOGIST John Schwabe Portland Donald A. Hull CONTENTS INTRODUCTION -- - ---- -- -- --- Purpose and Scope of this Report Acknowledgments U.S. Nickel Industry GEOLOGY OF LATERITE DEPOSITS - -- - 3 Previous Work - - - - --- 3 Ultramafic Rocks - ----- --- 3 Composition - - -------- - 3 Distribution ------ - - - 3 Structure - 3 Geochemistry of Nickel ---- 4 Chemical Weathering of Peridotite - - 4 The soi I profile ------- 5 M i nero I ogy -- - ----- 5 Prospecting Guides and Techniques- - 6 OTHER TYPES OF NICKEL DEPOSITS - - 7 Nickel Sulfide Deposits- - - - - - 7 Deposits in Oregon 7 Other areas --- 8 Prospecting techniques 8 Silica-Carbonate Deposits - -- 8 DISTRIBUTION OF LATERITE DEPOSITS - ------ 9 Nickel Mountain Deposits - - ------ --------- 9 Location --------------- --- 9 Geology - ------- ----- 11 Ore deposits ----------- - -- 11 Soil mineralogy - ------- 12 Structure --- ---- ---- 13 Mining and metallurgy ------------ ---- 13 Production- -

The Efficient Improvement of Original Magnetite in Iron Ore Reduction
minerals Article The Efficient Improvement of Original Magnetite in Iron Ore Reduction Reaction in Magnetization Roasting Process and Mechanism Analysis by In Situ and Continuous Image Capture Bing Zhao 1,2, Peng Gao 1,2,*, Zhidong Tang 1,2 and Wuzhi Zhang 1,2 1 School of Resources and Civil Engineering, Northeastern University, Shenyang 110819, China; [email protected] (B.Z.); [email protected] (Z.T.); [email protected] (W.Z.) 2 National-Local Joint Engineering Research Center of High-Efficient Exploitation Technology for Refractory Iron Ore Resources, Shenyang 110819, China * Correspondence: [email protected]; Tel.: +86-024-8368-8920 Abstract: Magnetization roasting followed by magnetic separation is considered an effective method for recovering iron minerals. As hematite and magnetite are the main concomitant constituents in iron ores, the separation index after the magnetization roasting will be more optimized than with only hematite. In this research, the mechanism of the original magnetite improving iron ore reduction during the magnetization roasting process was explored using ore fines and lump ore samples. Under optimum roasting conditions, the iron grade increased from 62.17% to 65.22%, and iron recovery increased from 84.02% to 92.02% after separation, when Fe in the original magnetite content increased from 0.31% to 8.09%, although the Fe masses in each sample were equal. For lump ores with magnetite and hematite intergrowth, the method of in situ and continuous image capture Citation: Zhao, B.; Gao, P.; Tang, Z.; for microcrack generation and the evolution of the magnetization roasting process was innovatively Zhang, W. -

Recovery of Magnetite-Hematite Concentrate from Iron Ore Tailings
E3S Web of Conferences 247, 01042 (2021) https://doi.org/10.1051/e3sconf/202124701042 ICEPP-2021 Recovery of magnetite-hematite concentrate from iron ore tailings Mikhail Khokhulya1,*, Alexander Fomin1, and Svetlana Alekseeva1 1Mining Institute of Kola Science Center of Russian Academy of Sciences, Apatity, 184209, Russia Abstract. The research is aimed at study of the probable recovery of iron from the tailings of the Olcon mining company located in the north-western Arctic zone of Russia. Material composition of a sample from a tailings dump was analysed. The authors have developed a separation production technology to recover magnetite-hematite concentrate from the tailings. A processing flowsheet includes magnetic separation, milling and gravity concentration methods. The separation technology provides for production of iron ore concentrate with total iron content of 65.9% and recovers 91.0% of magnetite and 80.5% of hematite from the tailings containing 20.4% of total iron. The proposed technology will increase production of the concentrate at a dressing plant and reduce environmental impact. 1 Introduction The mineral processing plant of the Olcon JSC, located at the Murmansk region, produces magnetite- At present, there is an important problem worldwide in hematite concentrate. The processing technology the disposal of waste generated during the mineral includes several magnetic separation stages to produce production and processing. Tailings dumps occupy huge magnetite concentrate and two jigging stages to produce areas and pollute the environment. However, waste hematite concentrate from a non-magnetic fraction of material contains some valuable components that can be magnetic separation [13]. used in various industries. In the initial period of plant operation (since 1955) In Russia, mining-induced waste occupies more than iron ore tailings were stored in the Southern Bay of 300 thousand hectares of lands. -

Banded Iron Formations
Banded Iron Formations Cover Slide 1 What are Banded Iron Formations (BIFs)? • Large sedimentary structures Kalmina gorge banded iron (Gypsy Denise 2013, Creative Commons) BIFs were deposited in shallow marine troughs or basins. Deposits are tens of km long, several km wide and 150 – 600 m thick. Photo is of Kalmina gorge in the Pilbara (Karijini National Park, Hamersley Ranges) 2 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock Iron rich bands: hematite (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). Iron poor bands: chert (fine‐grained quartz) and low iron oxide levels Rock sample from a BIF (Woudloper 2009, Creative Commons 1.0) Iron rich bands are composed of hematitie (Fe2O3), magnetite (Fe3O4), siderite (FeCO3) or pyrite (FeS2). The iron poor bands contain chert (fine‐grained quartz) with lesser amounts of iron oxide. 3 What are Banded Iron Formations (BIFs)? • Large sedimentary structures • Bands of iron rich and iron poor rock • Archaean and Proterozoic in age BIF formation through time (KG Budge 2020, public domain) BIFs were deposited for 2 billion years during the Archaean and Proterozoic. There was another short time of deposition during a Snowball Earth event. 4 Why are BIFs important? • Iron ore exports are Australia’s top earner, worth $61 billion in 2017‐2018 • Iron ore comes from enriched BIF deposits Rio Tinto iron ore shiploader in the Pilbara (C Hargrave, CSIRO Science Image) Australia is consistently the leading iron ore exporter in the world. We have large deposits where the iron‐poor chert bands have been leached away, leaving 40%‐60% iron. -

Smelting Iron from Laterite: Technical Possibility Or Ethnographic Aberration?
Smelting Iron from Laterite: Technical Possibility or Ethnographic Aberration? T. O. PRYCE AND S. NATAPINTU introduction Laterites deposits (orlateriticsoilsastheyarealsocalled)arefrequently reported in Southeast Asia, and are ethnographically attested to have been used for the smelting of iron in the region (Abendanon 1917 in Bronson 1992:73; Bronson and Charoenwongsa 1986), as well as in Africa (Gordon and Killick 1993; Miller and Van Der Merwe 1994). The present authors do not dispute this evidence; we merely wish to counsel cautioninitsextrapolation.Modifyingour understanding of a population’s potential to locally produce their own iron has immediate ramifications for how we reconstruct ancient metal distribution net- works, and the social exchanges that have facilitated them since iron’s generally agreed appearance in Southeast Asian archaeological contexts during the mid-first millennium b.c. (e.g., Bellwood 2007:268; Higham 1989:190). We present this paper as a wholly constructive critique of what appears to be a prevailingperspectiveonpre-modernSoutheastAsianironmetallurgy.Wehave tried to avoid technical language and jargon wherever possible, as our aim is to motivate scholars working within the regiontogivefurtherconsiderationtoiron as a metal, as a technology, and as a socially significant medium (e.g., Appadurai 1998; Binsbergen 2005; Gosden and Marshall 1999). When writing a critique it is of course necessary to cite researchers with whom one disagrees, and we have done this with full acknowledgment that in modern archaeology no one person can encompass the entire knowledge spectrum of the discipline.1 The archaeome- tallurgy of iron is probably on the periphery of most of our colleagues’ interests, but sometimes, within the technical, lies the pivotal, and in sharing some of our insights we hope to illuminate issues of benefit to all researchers in Metal Age Southeast Asia. -

ITP Mining: Energy and Environmental Profile of the U.S. Mining Industry: Chapter 4: Iron
Energy and Environmental Profile of the U.S. Mining Industry 4 Iron The chemical element iron is the fourth most common element in the Earth's crust and the second most abundant metal. About five percent of the Earth's crust is composed of iron. The metal is chemically active and is found in nature combined with other elements in rocks and soils. In its natural state, iron is chemically bonded with oxygen, water, carbon dioxide, or sulfur in a variety of minerals. Forms of Iron Minerals, Ores, and Rocks Iron occurs mainly in iron-oxide ores. Some ores are a mixture of minerals rich in iron. Other iron ores are less rich and have a large number of impurities. The most important iron ore- forming minerals are: • Magnetite - Magnetite (Fe3O4) forms magnetic black iron ore. There are large deposits of magnetite in Russia and Sweden. • Hematite - Hematite (Fe2O3) is a red iron ore. Hematite occurs in almost all forms, from solid rock to loose earth. It is the most plentiful iron ore and occurs in large quantities throughout the world. • Goethite - Goethite (Fe2O3.H2O), a brown ore, contains iron. • Limonite - Limonite (Fe2O3.H2O) is a yellow-brown iron ore. Limonite is a collective term for impure goethite and a mixture of hydrated iron oxides. Iron 4-1 Energy and Environmental Profile of the U.S. Mining Industry Taconite contains low-grade iron in fine specks and bands. It is an extremely hard and flinty - containing about 25 - 30 percent iron. The iron in taconite occurs principally as magnetite and hematite and finely dispersed with silica in sedimentary deposits. -

Mt Keith Presentation
Australian Site Tour Mt Keith Operation Jaco Harwig General Manager 28 October 2008 Important notices Reliance on third party information The views expressed here contain information that have been derived from publicly available sources that have not been independently verified. No representation or warranty is made as to the accuracy, completeness or reliability of the information. This presentation should not be relied upon as a recommendation or forecast by BHP Billiton. Forward looking statements This presentation includes forward-looking statements within the meaning of the U.S. Securities Litigation Reform Act of 1995 regarding future events and the future financial performance of BHP Billiton. These forward-looking statements are not guarantees or predictions of future performance, and involve known and unknown risks, uncertainties and other factors, many of which are beyond our control, and which may cause actual results to differ materially from those expressed in the statements contained in this presentation. For more detail on those risks, you should refer to the sections of our annual report on Form 20- F for the year ended 30 June 2008 entitled “Risk factors” , “Forward looking statements” and “Operating and financial review and prospects” filed with the U.S. Securities and Exchange Commission. No offer of securities Nothing in this release should be construed as either an offer to sell or a solicitation of an offer to buy or sell BHP Billiton securities in any jurisdiction. Cautionary Note to US Investors The SEC generally permits mining companies in their filings with the SEC to disclose only those mineral deposits that the company can economically and legally extract. -

Conservation and Systematic Development of Iron
1 PART : II INTRODUCTION CONSERVATION (PRESERVATION) AND SYSTEMATIC DEVELOPMENT OF IRON ORE One of the species of National Wealth is iron ore. Conservation of this mineral is of prime importance for industrial development. Indian legislature has recognized it in Section: 18 of the Mines & Minerals (Development & Regulation) Act, 1957. Section : 18, inter-alia, directs the Central Government to take all such steps as may be necessary for: (a) the conservation (Preservation) (b) and systematic development of minerals in India Systematic development of minerals in India would require: (a) exact estimate of reserves available (b) reasonable estimate of resources from where probable reserves can be estimated. This, inter–alia, would require opening of new mines. For that purpose, exploration is necessary. 2 For this, it would be worthwhile to refer to: A (REPORTS FOR EXPLORATION) (i) DOCUMENT ON STRATEGY FOR EXPLORATION, EXPLOITATION AND DEVELOPMENT FOR IRON ORE IN INDIA, PUBLISHED BY THE SUB–GROUP ON IRON ORE in India (January, 2006) (ii) MINERAL POLICY ISSUES IN THE CONTEXT OF EXPORT AND DOMESTIC USE OF IRON ORE IN INDIA - REPORT - FEBRUARY 2008 (INDIAN COUNCIL FOR RESEARCH ON INTERNATIONAL ECONOMIC RELATIONS) (iii) ECONOMICS OF SPONGE IRON AND STEEL PRODUCTION (SEPTEMBER, 2008) BY STEEL AND NATURAL RESOURCES STRATEGY RESEARCH, VASANT KUNJ, NEW DELHI (iv) IRON ORE – STATUS AND FUTURE PROSPECTS” by M. S. Jairam, Director, Geological Survey of India (v) IBM REPORT, 2009, AND PROVISIONAL FIGURES, AS ON 1-4-2010, SUPPLIED BY IBM, FOR IRON ORE 3 B (ILLEGAL MINING) (vi) 19TH REPORT OF STANDING COMMITTEE ON COAL & STEEL which highlights menace of illegal mining.