Interspecific Hybridization and Restricted Trans-Pacific Gene Flow In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taxonomic Checklist of CITES Listed Coral Species Part II
CoP16 Doc. 43.1 (Rev. 1) Annex 5.2 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of CITES listed Coral Species Part II CORAL SPECIES AND SYNONYMS CURRENTLY RECOGNIZED IN THE UNEP‐WCMC DATABASE 1. Scleractinia families Family Name Accepted Name Species Author Nomenclature Reference Synonyms ACROPORIDAE Acropora abrolhosensis Veron, 1985 Veron (2000) Madrepora crassa Milne Edwards & Haime, 1860; ACROPORIDAE Acropora abrotanoides (Lamarck, 1816) Veron (2000) Madrepora abrotanoides Lamarck, 1816; Acropora mangarevensis Vaughan, 1906 ACROPORIDAE Acropora aculeus (Dana, 1846) Veron (2000) Madrepora aculeus Dana, 1846 Madrepora acuminata Verrill, 1864; Madrepora diffusa ACROPORIDAE Acropora acuminata (Verrill, 1864) Veron (2000) Verrill, 1864; Acropora diffusa (Verrill, 1864); Madrepora nigra Brook, 1892 ACROPORIDAE Acropora akajimensis Veron, 1990 Veron (2000) Madrepora coronata Brook, 1892; Madrepora ACROPORIDAE Acropora anthocercis (Brook, 1893) Veron (2000) anthocercis Brook, 1893 ACROPORIDAE Acropora arabensis Hodgson & Carpenter, 1995 Veron (2000) Madrepora aspera Dana, 1846; Acropora cribripora (Dana, 1846); Madrepora cribripora Dana, 1846; Acropora manni (Quelch, 1886); Madrepora manni ACROPORIDAE Acropora aspera (Dana, 1846) Veron (2000) Quelch, 1886; Acropora hebes (Dana, 1846); Madrepora hebes Dana, 1846; Acropora yaeyamaensis Eguchi & Shirai, 1977 ACROPORIDAE Acropora austera (Dana, 1846) Veron (2000) Madrepora austera Dana, 1846 ACROPORIDAE Acropora awi Wallace & Wolstenholme, 1998 Veron (2000) ACROPORIDAE Acropora azurea Veron & Wallace, 1984 Veron (2000) ACROPORIDAE Acropora batunai Wallace, 1997 Veron (2000) ACROPORIDAE Acropora bifurcata Nemenzo, 1971 Veron (2000) ACROPORIDAE Acropora branchi Riegl, 1995 Veron (2000) Madrepora brueggemanni Brook, 1891; Isopora ACROPORIDAE Acropora brueggemanni (Brook, 1891) Veron (2000) brueggemanni (Brook, 1891) ACROPORIDAE Acropora bushyensis Veron & Wallace, 1984 Veron (2000) Acropora fasciculare Latypov, 1992 ACROPORIDAE Acropora cardenae Wells, 1985 Veron (2000) CoP16 Doc. -

Bleaching and Recovery of Five Eastern Pacific Corals in an El Niño-Related Temperature Experiment
BULLETIN OF MARINE SCIENCE, 69(1): 215–236, 2001 BLEACHING AND RECOVERY OF FIVE EASTERN PACIFIC CORALS IN AN EL NIÑO-RELATED TEMPERATURE EXPERIMENT Christiane Hueerkamp, Peter W. Glynn, Luis D’Croz, Juan L. Maté and Susan B. Colley ABSTRACT Coral bleaching events have increased in frequency and severity, due mainly to el- evated water temperature associated with El Niño-related warming and a general global warming trend. We experimentally tested the effects of El Niño-like sea temperature conditions on five reef-building corals in the Gulf of Panama. Branching species (Pocillopora damicornis and Pocillopora elegans) and massive species (Porites lobata, Pavona clavus and Pavona gigantea) were exposed to experimentally elevated seawater temperature, ~1–2∞C above ambient. Differences in zooxanthellate coral responses to bleaching and ability to recover were compared and quantified. All corals exposed to high temperature treatment exhibited significant declines in zooxanthellae densities and chlorophyll a concentrations. Pocilloporid species were the most sensitive, being the first to bleach, and suffered the highest mortality (50% after 50 d exposure). Massive coral species demonstrated varying tolerances, but were generally less affected. P. gigantea exhibited the greatest resistance to bleaching, with no lethal effects observed. Maximum experimental recovery was observed in P. lobata. No signs of recovery occurred in P. clavus, as zooxanthellae densities and chlorophyll a concentrations continued to decline under ambient (control) conditions. Experimental coral responses from populations in an upwelling environment are contrasted with field responses observed in a nonupwelling area during the 1997–98 El Niño–Southern Oscillation event. A variety of stressors can lead to coral bleaching, the whitening of coral tissue, a prob- lem that threatens reefs worldwide. -

STAFF WORKING PAPER SUMMARY of SELECTED PEARL HARBOR MARINE NATURAL RESOURCES DATA from 1999 – 2015 - in SUPPORT of PROPOSED PROJECT P 516 Prepared by Stephen H
1 STAFF WORKING PAPER SUMMARY OF SELECTED PEARL HARBOR MARINE NATURAL RESOURCES DATA FROM 1999 – 2015 - IN SUPPORT OF PROPOSED PROJECT P 516 Prepared by Stephen H. Smith Marine Ecologist SSC Scientific Diving Services March 18, 2015 Introduction Overview. The objective of this Staff Working Paper is to summarize selected data gathered by the author between 1999 and February 2015. During that time period, the author conducted a variety of assessments throughout Pearl Harbor and the Pearl Harbor Entrance Channel. The specific resources which will be addressed in this partial summary are: 1) corals, 2) selected fin fish species and Essential Fish Habitat (EFH), 3) sea turtles, 4) miscellaneous and 5) perceived data gaps. This summary is not intended to reiterate material already presented in the Pearl Harbor INRMP or the many other documents which contain pertinent marine natural resource data; it is intended to summarize unpublished and/or unreported data gathered by the author. In this document, Pearl Harbor is defined as the area north of Hammer Point, as designated on Nautical Chart No. 19366 (Oahu South Coast Pearl Harbor). The Pearl Harbor Entrance Channel (PHEC) is defined as the area south of Hammer Point between the channel markers on the eastern and western sides of the PHEC and extending to the outermost Channel Marker Buoys (No. 1 on the west side and No. 2 on the east side). Figure 1 illustrates the boundaries of the P 516 project assessment area. All the data summarized in this document was gathered by the author, with periodic biological support from Donald Marx, and others. -

Scleractinian Reef Corals: Identification Notes
SCLERACTINIAN REEF CORALS: IDENTIFICATION NOTES By JACKIE WOLSTENHOLME James Cook University AUGUST 2004 DOI: 10.13140/RG.2.2.24656.51205 http://dx.doi.org/10.13140/RG.2.2.24656.51205 Scleractinian Reef Corals: Identification Notes by Jackie Wolstenholme is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. TABLE OF CONTENTS TABLE OF CONTENTS ........................................................................................................................................ i INTRODUCTION .................................................................................................................................................. 1 ABBREVIATIONS AND DEFINITIONS ............................................................................................................. 2 FAMILY ACROPORIDAE.................................................................................................................................... 3 Montipora ........................................................................................................................................................... 3 Massive/thick plates/encrusting & tuberculae/papillae ................................................................................... 3 Montipora monasteriata .............................................................................................................................. 3 Massive/thick plates/encrusting & papillae ................................................................................................... -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -

Evaluación De Riesgo De Extinción De Pocillopora Inflata De Acuerdo Al Numeral 5.7 De La Norma Oficial Mexicana NOM-059-SEMARNAT-2010
Evaluación de Riesgo de Extinción de Pocillopora inflata de acuerdo al numeral 5.7 de la Norma Oficial Mexicana NOM-059-SEMARNAT-2010. 5.7.1 DATOS GENERALES DEL RESPONSABLE DE LA PROPUESTA Autores: Nájera-Hillman Eduardo1 y Meléndez-Rosas Rebeca1 1COSTASALVAJE, A.C. Domicilio: Boulevard Las Dunas No. 160 Interior 203. Fraccionamiento Playa de Ensenada, Ensenada Baja California. CP 22880 Teléfono: 016461521518 e-mail: [email protected], [email protected] Asesores: 5.7.2 NOMBRE CIENTÍFICO VÁLIDO CITANDO LA AUTORIDAD TAXONÓMICA RESPECTIVA Pocillopora inflata Glynn, 1999 La página web utilizada para proporcionar el nombre científico de la especie, fue WoRMS (WoRMS editorial board, 2018) y UICN-Red List for Threatened Species. SINÓNIMOS No existen NOMBRES COMUNES No existen TAXONOMÍA Reino: Animalia Phylum: Cnidaria Clase: Anthozoa Orden: Scleractinia Familia: Pocilloporidae Genero: Pocillopora Especie: Pocillopora inflata Glynn, 1999 1 2 1Fotografía: Glynn, 1999. 2Fotografía: Reyes-Bonilla et al. 2017, tomada al Sur del Golfo de California. MOTIVO DE LA PROPUESTA La investigación documental que se condujo sobre la especie de coral P. inflata, evidenció el riesgo que tiene esta especie de desaparecer del territorio nacional. Debido a su restringido rango de distribución, prácticas de explotación comercial por el acuarismo y extensas amenazas al hábitat de arrecifes de coral, se propone que la especie sea incorporada bajo la categoría “Amenazada” de la NOM-059- SEMARNAT-2010. 5.7.3 MAPA DE DISTRIBUCIÓN GEOGRÁFICA DE P. inflata Figura 1. Registros de la distribución del coral Pocillopora inflata en México. 5.7.4 JUSTIFICACIÓN TÉCNICA CIENTÍFICA DE LA PROPUESTA a) Análisis diagnóstico del estado actual de la especie y su hábitat El coral Pocillopora inflata tiene una distribución geográfica restringida, siendo endémico del Pacífico Oriental Tropical. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -

Development of Coral and Zooxanthella-Specific
Development of coral and zooxanthella-specific microsatellites in three species of Pocillopora (Cnidaria, Scleractinia) from French Polynesia Hélène Magalon, Sarah Samadi, Murielle Richard, Mehdi Adjeroud, Michel Veuille To cite this version: Hélène Magalon, Sarah Samadi, Murielle Richard, Mehdi Adjeroud, Michel Veuille. Development of coral and zooxanthella-specific microsatellites in three species of Pocillopora (Cnidaria, Scleractinia) from French Polynesia. Molecular Ecology Notes, Wiley-Blackwell, 2004, 4, pp.206-8. hal-00941708 HAL Id: hal-00941708 https://hal.archives-ouvertes.fr/hal-00941708 Submitted on 6 May 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Blackwell Publishing, Ltd. Development of coral and zooxanthella-specific microsatellites in three species of Pocillopora (Cnidaria, Scleractinia) from French Polynesia HÉLÈNE MAGALON,* SARAH SAMADI,*‡ MURIELLE RICHARD,* MEHDI ADJEROUD† and MICHEL VEUILLE* *Ecole Pratique des Hautes Etudes/UMR CNRS 7625, laboratoire d’Ecologie, Université Pierre et Marie Curie, 7 quai saint Bernard, 75005 Paris, France, †Ecole Pratique des Hautes Etudes, Laboratoire de Biologie Marine et Malacologie, UMR CNRS 8046, Université de Perpignan, 66860 Perpignan, France Abstract Since the building of coral reefs results from the association of corals and zooxanthellae, their intracellular algal symbionts, genetic markers for both organisms are essential for studying the contribution of their respective dispersal to the resilience of endangered reef ecosystems. -

Index to Volume 53
Index to Volume 53 Author Index ABURTO-OROPEZA, OCTAVIo-see Sala et al. EWING, CURTIS ALLEN, MELINDA S., and GAIL M. MURAKAMI The Endemic Hawaiian Sap Beetles (Coleoptera: Liina'i Island's Arid Lowland Vegetation in Late Nitidulidae): Distribution, Ecological Shifts, and Prehistory, 88-112 Adaptation (abstract), 114-115 ARREOLA-RoBLES, JosE L.-see Sala et al. ASQUITH, ADAM-see Kido et al. ATAN, HUGO-see Osorio et al. FILARDI, CHRISTOPHER E., CATHERINE E. SMITH, ATKINSON, MARLIN J.-see Tarrant et al. ANDREW W. KRATTER, DAVID W. STEADMAN, and ATKINSON, SHANNON-See Tarrant et al. H. PRICE WEBB New Behavioral, Ecological, and Biogeographic Data BAILEy-BROCK, JULIE H. on the Avifauna of Rennell, Solomon Islands, 319 NeriIlidae of Hawai'i: Two New Records of 340 Interstitial Polychaetes, 299-304 FLETCHER, CHARLES H., III-see Harney et al. see also Brock et al. FOALE, SIMON BAILEy-BROCK, JULIE H., VERNON R. BROCK, and Local Ecological Knowledge and Biology of the Land RiCHARD E. BROCK Crab Cardisoma hirtipes (Decapoda: Gecarcinidae) Intrusion of Anchialine Species in the Marine at West Nggela, Solomon Islands, 37-49 Environment: The Appearance of an Endemic FOSTER, JAMIE S. Hawaiian Shrimp, Halocaridina rubra, on the South Lipopolysaccharide-Induced Apoptosis in the Shore ofO'ahu (Hawaiian Islands), 367-369 Symbiotic Light Organ of the Sepiolid Squid BIRKELAND, C. E.-see Green et al. Euprymna scolopes (abstract), liS BROCK, RICHARD E.-see Bailey-Brock et al. BROCK, RiCHARD, JULIE H. BAILEy-BROCK, and JOHN GARB, JESSICA E. GOODY Origins ofthe Hawaiian Crab Spider Fauna (abstract), A Case Study of Efficacy of Freshwater Immersion in 115-116 Controlling Introduction of Alien Marine Fouling GARRISON, JENNIFER Communities: The USS Missouri, 223-231 Role of Alien Tree Plantations in Native Forest BROCK, VERNON R.-see Bailey-Brock et al. -

FDM 2017 Coral Species Reef Survey
Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific Final ® FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT Dr. Jessica Carilli, SSC Pacific Mr. Stephen H. Smith, SSC Pacific Mr. Donald E. Marx Jr., SSC Pacific Dr. Leslie Bolick, SSC Pacific Dr. Douglas Fenner, NOAA August 2018 Prepared for U.S. Navy Pacific Fleet Commander Pacific Fleet 250 Makalapa Drive Joint Base Pearl Harbor Hickam Hawaii 96860-3134 Space and Naval Warfare Systems Center Pacific Technical Report number 18-1079 Distribution Statement A: Unlimited Distribution 1 Submitted in support of the U.S. Navy’s 2018 Annual Marine Species Monitoring Report for the Pacific REPORT DOCUMENTATION PAGE Form Approved OMB No. 0704-0188 Public reporting burden for this collection of information is estimated to average 1 hour per response, including the time for reviewing instructions, searching data sources, gathering and maintaining the data needed, and completing and reviewing the collection of information. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden to Washington Headquarters Service, Directorate for Information Operations and Reports, 1215 Jefferson Davis Highway, Suite 1204, Arlington, VA 22202-4302, and to the Office of Management and Budget, Paperwork Reduction Project (0704-0188) Washington, DC 20503. PLEASE DO NOT RETURN YOUR FORM TO THE ABOVE ADDRESS. 1. REPORT DATE (DD-MM-YYYY) 2. REPORT TYPE 3. DATES COVERED (From - To) 08-2018 Monitoring report September 2017 - October 2017 4. TITLE AND SUBTITLE 5a. CONTRACT NUMBER FARALLON DE MEDINILLA 2017 SPECIES LEVEL CORAL REEF SURVEY REPORT 5b. -

Molecular Phylogenetics of Trapezia Crabs in the Central Mexican Pacific
Article Molecular Phylogenetics of Trapezia Crabs in the Central Mexican Pacific Hazel M. Canizales-Flores, Alma P. Rodríguez-Troncoso *, Eric Bautista-Guerrero and Amílcar L. Cupul-Magaña Laboratorio de Ecología Marina, Centro Universitario de la Costa, Universidad de Guadalajara, Av. Universidad No. 203. Puerto Vallarta, Jalisco 48280, Mexico; [email protected] (H.M.C.-F.); [email protected] (E.B.-G.); [email protected] (A.L.C.-M.) * Correspondence: [email protected]; Tel.: +52-322-226-2319 Received: 15 July 2020; Accepted: 24 August 2020; Published: 26 August 2020 Abstract: To date, Trapezia spp. crabs have been considered obligate symbionts of pocilloporid corals. They protect their coral hosts from predators and are essential for the health of certain coral species. However, the basic details of this group of crustaceans are lacking, and there is a need for species-level molecular markers. The Tropical Eastern Pacific (TEP) region harbors important coral communities mainly built by corals of the genus Pocillopora, with three known Trapezia species known to associate with them: Trapezia bidentata, T. formosa and T. corallina. Both taxonomic and molecular analyses were carried out with samples of all three crab species collected from Pocillopora spp. in the Central Mexican Pacific. Analysis of both a mitochondrial and a nuclear gene revealed only two species, T. corallina and T. bidentata. T. formosa however appears to be a morphotype of T. bidentata. The use of integrative taxonomy for this group has increased the knowledge of the biodiversity not only of the study area, but of the whole TEP and will enhance the future study of the Trapezia–Pocillopora symbiosis. -
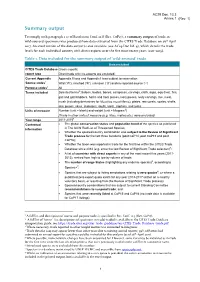
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g.