Total Mercury Levels in Invasive Lionfish, <I>Pterois Volitans</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Documenting the Invasion of Lionfish in Florida's Waters And
Documenting the Invasion of Lionfish in Florida’s Waters and Management Efforts to Control Them Image credit: Image Bryan Fluech Scorpionfish Family Two visually identical species found in the Southeastern U.S. Red Lionfish (Pterois volitans) Devil Firefish (Pterois miles) Image credits: www.lionfishhunters.org Native Distribution Red Lionfish – Pterois volitans Devil Firefish – Pterois miles Location of Venomous Spines 13 2 3 Venomology Image Image credit: REEF Lionfish venom is a protein based neurotoxin and is contained in glandular venom tissue in grooves along either side of each spine Image credit: Dawn Witherington Lionfish Envenomation Pain is immediate; intensifies “It won’t kill you, but it’ll over 60-90 minutes; may last make you wish you were 6-12 hours dead.” Treatment involves covering the wound with hot (not scalding) water; use of over-the-counter pain relievers Victims should seek medical attention Other symptoms might include ulceration of the wound site, headaches, nausea or diarrhea Image Credit: Roxane Boonstra Lionfish Invasion First record : 1985 in Dania, FL Aquarium releases or escapes are most likely sources of the invasion Over 60,000 imported into Florida annually* Mitochondrial data show no evidence of multiple independent introductions Documenting the Invasion Image credit: USGS Lionfish Issues Image credit: Florida Sea Grant Highly Productive Year round reproduction Can spawn every 2-4 days 20,000 – 30,000 eggs / spawn Larvae dispersed by currents Sexually mature within a year Egg Mass Image -

The Invasion of Indo-Pacific Lionfish in the Bahamas: Challenges for a National Response Plan
The Invasion of Indo-Pacific Lionfish in the Bahamas: Challenges for a National Response Plan KATHLEEN SULLIVAN SEALEY1, 4, LAKESHIA ANDERSON2, DEON STEWART3, and NICOLA SMITH4, 5 1University of Miami Department of Biology, Coral Gables, Florida USA; 2 Department of Marine Resources, Nassau, Bahamas; 3 Bahamas Environment, Science and Technology Commission, Nassau, Bahamas; 4 Marine and Environmental Studies Institute, College of The Bahamas, Nassau, Bahamas; 5 Department of Zoology, University of British Columbia, Vancouver, BC, Canada ABSTRACT The invasion of the Indo-Pacific lionfish to Bahamian waters raises considerable concern due to the uncertainty of its ecological impacts and its potential threats to commercial fisheries and human safety. Lionfish have been reported throughout the archipelago and are the focus of several research and monitoring initiatives. The Bahamas has a National Invasive Species Strategy, but marine invasions require unique partnerships for small islands developing states to develop realistic management goals and actions. The Government of The Bahamas has limited funds to address major resource management issues; hence, collaboration with non-governmental agencies and tertiary education institutions is imperative. The establishment and spread of lionfish has created a novel opportunity for the formation of innovative public-private partnerships to address the ecological, economic and social impacts of biological invasions. KEY WORDS: Lionfish, invasion, reefs La Invasión en las Bahamas del Pez León del Indo-Pacifico: Un Caso de Investigación, Planificación, y Manejo La invasión del pez león del Indopacífico en las aguas de Las Bahamas ha generado una gran preocupación debido a la incertidumbre sobre su efecto ecológico y la posible afectación a la pesca comercial, el turismo y la seguridad de la población. -

A Guide to Harmful and Toxic Creatures in the Goa of Jordan
Published by the Royal Marine Conservation Society of Jordan. P. O. Box 831051, Abdel Aziz El Thaalbi St., Shmesani 11183. Amman Copyright: © The Royal Marine Conservation Society of Jordan Reproduction of this publication for educational and other non- commercial purposes is authorized without prior written approval from the copyright holder provided the source is fully acknowledged. ISBN: 978-9957-8740-1-8 Deposit Number at the National Library: 2619/6/2016 Citation: Eid, E and Al Tawaha, M. (2016). A Guide to Harmful and Toxic Creature in the Gulf of Aqaba of Jordan. The Royal Marine Conservation Society of Jordan. ISBN: 978-9957-8740-1-8. Pp 84. Material was reviewed by Dr Nidal Al Oran, International Research Center for Water, Environment and Energy\ Al Balqa’ Applied University,and Dr. Omar Attum from Indiana University Southeast at the United State of America. Cover page: Vlad61; Shutterstock Library All photographs used in this publication remain the property of the original copyright holder, and it should not be reproduced or used in other contexts without permission. 1 Content Index of Creatures Described in this Guide ......................................................... 5 Preface ................................................................................................................ 6 Part One: Introduction ......................................................................................... 8 1.1 The Gulf of Aqaba; Jordan ......................................................................... 8 1.2 Aqaba; -
Field Identification Guide to the Living Marine Resources in Kenya
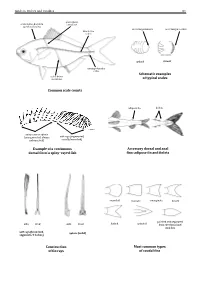
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Pterois Miles (Devil Firefish Or Common Lionfish)
UWI The Online Guide to the Animals of Trinidad and Tobago Diversity Pterois miles (Devil Firefish or Common Lionfish) Family: Scorpaenidae (Scorpionfish) Order: Scorpaeniformes (Mail-cheeked Fish) Class: Actinopterygii (Ray-finned fishes) Fig. 1. Devil firefish, Pterois miles. [http://pre.apaaweb.com/jordania_2009.html, downloaded 8 October 2016] TRAITS. The devil firefish or common lionfish Pterois miles has maroon and white coloured stripes throughout its entire body that allows them to blend into some habitats; it has elaborate dorsal, pelvic and pectoral fins and a pair of tentacles above its eyes (Fig. 1). It has cycloid (rounded) scales (Belmore, 2013), and the head is large, up to one third its body length. The firefish has 10 dorsal and 6 anal fin spines, all with venom that is produced from the enclosing skin (Schofield et al., 2012). The caudal (tail) rays are not enclosed by venom-producing skin. Males and females look exactly alike except during times of mating (Morris et al., 2009). Adults typically grow up to 30cm in length, but can reach 43cm and weigh 1.1kg (Belmore, 2013). DISTRIBUTION. The native geographical area of Pterois miles is mainly in the Indian Ocean (Fig. 2). More specifically it is found from the Indochina Peninsula to India and the Red Sea (Belmore, 2013). However, the devil firefish has been introduced and invaded other far-reaching regions of the world such as the east coast of the USA and Caribbean waters (Morris, 2009), and the Red Sea, probably migrating through the Suez Canal (Schofield et al., 2012). This species invaded Tobago’s waters a few years ago, at the tail end of the Caribbean, demonstrating its continued migration. -

Competitive Interactions for Shelter Between Invasive Pacific Red Lionfish and Native Nassau Grouper
Environ Biol Fish DOI 10.1007/s10641-014-0236-9 Competitive interactions for shelter between invasive Pacific red lionfish and native Nassau grouper Wendel W.Raymond & Mark A. Albins & Timothy J. Pusack Received: 12 September 2013 /Accepted: 14 January 2014 # Springer Science+Business Media Dordrecht 2014 Abstract The invasive Pacific red lionfish (Pterois positioned closer to and used limited shelter more when volitans) poses a threat to western Atlantic and Carib- paired with similarly sized lionfish and (2) grouper bean coral reef systems. Lionfish are small-bodied pred- moved much further away from shelter when paired ators that can reduce the abundance and diversity of with smaller lionfish. We also found that neither large native fishes via predation. Additionally, native preda- lionfish nor large Nassau grouper preyed upon smaller tors or competitors appear to have a negligible effect on individuals of the opposite species suggesting that Nas- similarly sized lionfish. Nassau grouper (Epinephelus sau grouper do not recognize small lionfish as prey. This striatus) are a regionally endangered, large predator study highlights how invasive lionfish may affect native found throughout lionfish’s invasive range. Because Nassau grouper, and suggests that competition for shel- lionfish and Nassau grouper occupy similar habitats ter between these two species may be size dependent. and use similar resources, there is potential for compe- tition between these two species. Using large, outdoor Keywords Avoidance . Bahamas . Refuge . Shelter in-ground tanks, we investigated how lionfish and Nas- dominance . Size dependence sau grouper affect each other’s behavior by comparing their distance from and use of shelter when in isolation versus when both species were in the presence of each Introduction other with limited shelter. -

Pterois Miles) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Devil Firefish (Pterois miles) Ecological Risk Screening Summary Web Version—07/28/2014 Photo: © J.E. Randall from EOL (2014). 1 Native Range, and Status in the United States Native Range From Eschmeyer (1986): “Indian Ocean: Red Sea south to Port Alfred, South Africa and east to Sumatra, Indonesia (Fricke 1999).” Pterois miles Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version – 7/28/2014 Status in the United States From Schofield et al. (2014): “Atlantic Coast of USA: Lionfishes have been established from Miami to North Carolina since 2002. They established in the Florida Keys in 2009. Although present in Atlantic waters north of North Carolina, they are not likely to survive cold winter temperatures.” “Gulf of Mexico: Other than the anomalous Treasure Island specimen (see Schofield 2010), the first confirmed specimens of lionfish taken from the Gulf of Mexico were in December 2009. Sightings of lionfishes are becoming common in the northern Gulf of Mexico, especially associated with [artificial] reefs (including oil/gas platforms).” “Greater Antilles: Lionfishes are established off all islands in the Greater Antilles (Cuba [2007], Jamaica [2008], Hispañola [Haiti and the Dominican Republic; 2008] and Puerto Rico [2009]).” “Lesser Antilles: Lionfish presence has been confirmed throughout the leeward and windward islands. For more details, see Schofield (2010).” Means of Introductions in the United States From Schofield et al. (2014): “The most probable explanation for the arrival of lionfishes in the Atlantic Ocean is via the aquarium trade (Whitfield et al. 2002; Semmens et al. 2004). No one will ever know with certainty how lionfishes gained entry to the coastal waters of the U.S.; however, as they are common aquarium fishes, it is possible they were released pets. -

Risk Assessment for the Lionfish Pterois Miles (Bennett, 1828)
Risk Assessment for the lionfish Pterois miles (Bennett, 1828) Prepared in the framework of the EU LIFE project “Preventing a LIONfish invasion in the MEDiterranean through early response and targeted REmoval (RELIONMED-LIFE)” - LIFE16 NAT/CY/000832 EU NON-NATIVE ORGANISM RISK ASSESSMENT SCHEME Name of organism: Pterois miles (Bennett, 1828) Lead authors: Periklis Kleitou1, Ioannis Savva2, Demetris Kletou2 Contributing authors: Charalampos Antoniou2, Niki Chartosia3, Yiannis Christodoulides4,5, Maria Christou2, Louis Hadjioannou4, Margarita Hadjistylli5, Jason M Hall-Spencer1, Carlos Jimenez4, Sian Rees1 1 School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK 2 Marine and Environmental Research (MER) Lab., Limassol, 4533, Cyprus 3 Department of Biological Sciences University of Cyprus P.O. Box 20537, 1678 Nicosia, Cyprus. 4 Enalia Physis Environmental Research Centre, Acropoleos 2, Aglantzia 2101, Nicosia, Cyprus 5 Department of Environment, Ministry of Agriculture, Rural Development and Environment, Nicosia, Cyprus Risk Assessment Area: The geographical coverage of the risk assessment is the territory of the European Union (excluding outermost regions) and the United Kingdom Peer review 1: Stelios Katsanevakis, Dr, Department of Marine Sciences, University of the Aegean, Mytilene, Lesvos Island, Greece Peer review 2: Ernesto Azzurro, Dr, Institute for Environmental Protection and Research (ISPRA) Livorno, Italy Positive opinion by the Scientific Forum: 17/11/2020 1 | P a g e EU CHAPEAU QUESTION RESPONSE COMMENT -

New Mediterranean Biodiversity Records (July 2016) T
Collective Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net DOI: 10.12681/mms.1734 New Mediterranean Biodiversity Records (July 2016) T. DAILIANIS1,2, O. AKYOL3, N. BABALI4, M. BARICHE5, F. CROCETTA6, V. GEROVASILEIOU1,2, R. GHANEM7,8, M. GÖKOĞLU9, T. HASIOTIS2, A. IZQUIERDO-MUÑOZ10, D. JULIAN9, S. KATSANEVAKIS2, L. LIPEJ11, E. MANCINI12 , CH. MYTILINEOU6, K. OUNIFI BEN AMOR7,8, A. ÖZGÜL3, M. RAGKOUSIS2, E. RUBIO-PORTILLO13 , G. SERVELLO14 , M. SINI2, C. STAMOULI6, A. STERIOTI1, S. TEKER9, F. TIRALONGO12 and D. TRKOV11 1 Hellenic Centre for Marine Research, Institute of Marine Biology, Biotechnology, and Aquaculture, 71500 Heraklion Crete, Greece 2 University of the Aegean, Department of Marine Sciences, University Hill, 81100 Mytilene, Greece 3 Ege University, Faculty of Fisheries, 35440, Urla, Izmir, Turkey 4 National Research Center for the Developing of Fisheries and Aquaculture (CNRDPA), Algeria 5 American University of Beirut, Department of Biology, PO Box 11-0236, Beirut 1107 2020, Lebanon 6 Hellenic Centre for Marine Research, Institute of Marine Biological Resources and Inland Waters, 19013 Anavyssos, Greece 7 Département des Ressources Animales, Halieutiques et des Technologies Agroalimentaires, Institut National Agronomique de Tunisie, Université de Carthage, Tunis, Tunisia 8 Laboratoire de Biodiversité, Biotechnologies et Changements climatiques, Faculté des Sciences de Tunis, Université Tunis El Manar, Tunis, Tunisia -

Helium/Oxygen Breathing Gas Mixtures and Possibly! 3! High
EFFECTS OF LISTENING EXPERIENCE ON DECODING SPEECH IN HKLIOX ENVIRONMENTS Harry Hollien, Ph.D. Carl L Thompson,Ph. D. IPCP andLinguistics Universityof Florida Gainesville,FLORIDA 32611 U. S A. Veteran'sAdministration Medical Center, NewOrleans, LOUISIANA 70146U.SA. Thepurposeofthis experiment wasto assess 1!the ability of auditors todecode speechproduced inthe He02 envimnment and2! the effects oflistening experience uponthis skill/ability. Three pai red groups ofauditors listened toequated speech tasksand were tested on their ability to decode the heard utterances. The samples wereproduced bydivers situated inan underwater habitat atdepths upto 1000fsw Ofeach pair, one listener group was subjected todaily exposure ofHe02 speech samples no feedback! for twoweeks; the second group received no exposure. Findingsdemonstrated thatnormally hearing adults can decode about 25% of He02speech asheard and that some iru&iduals are much more adept at the task thanare others. Second, it was found that a simpleexposure to He02 speech resultedin a neardoubling of decoding ability and familiarity with the talkers furtherenhanced this capability. INTRODUCTION Asmay be seen in Figure 1,it isnow well established thatthe combined effects ofthe 1!high ambient pressures, 2!helium/oxygen breathing gasmixtures and possibly! 3!high pressurenervous snyndrome, associated withsaturation diving, lead to severely distorted speech Bennett, 1967; Brauer, 1982; Brauer, etal, 1966; Fant and Sonnesson, 1964;Fant and Lindquist,1968; Fant, et al, 1971; Flower, 1969; Gelfand, -

The Ecological and Socio-Economic Impacts of the Lionfish Invasion in the Southern Caribbean
UNIVERSITY OF SOUTHAMPTON FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Science Volume I of I The ecological and socio-economic impacts of the lionfish invasion in the Southern Caribbean by Fadilah Zafirah Ali Thesis for the degree of M.Phil September 2017 UNIVERSITY OF SOUTHAMPTON ABSTRACT FACULTY OF NATURAL AND ENVIRONMENTAL SCIENCES Ocean and Earth Science Thesis for the degree of Master of Philosophy THE ECOLOGICAL AND SOCIO-ECONOMIC IMPACTS OF THE LIONFISH INVASION IN THE SOUTHERN CARIBBEAN Fadilah Zafirah Ali The Indo-Pacific lionfish (Pterois volitans) is a venomous, voracious predator with a high dispersal capactity. In the space of 30 years, it has infiltrated an array of habitats, inhabited a depth range of > 300 m and exceeded the size and density reported in the native range, demonstrating the difficulty of effective lionfish management. If left unmanaged, lionfish pose a significant, but still uncertain, threat to Caribbean ecosystems thereby warranting the need for effective and efficient, tailored management schemes. Since their confirmation in Bonaire and Curacao in October 2009, an extensive monitoring program was established by the author in collaboration with CIEE Research Station Bonaire whereby >11,000 lionfish were documented and their population dynamics, reproductive and feeding ecology analysed in relation to local management strategies. The types of education and management strategies applied were evaluated based on their benefits and limitations to make recommendations for areas early in the invasion timeline. Finally efficiency of removal activities in Bonaire and Curacao were assessed and suggestions made on when and how often to remove lionfish. Socio-economic questionnaires were conducted to determine the profile and motivations of lionfish hunters, and a cost-benefit-analysis performed to assess economic effects of the invasion. -

Worcester Polytechnic Institute Major Qualifying Project Lionfish Bot 2.0 International Studies Report
Worcester Polytechnic Institute Major Qualifying Project Lionfish Bot 2.0 International Studies Report Submitted By: Thomas Ralph, Electrical Engineering & International Studies Advised By: Professor Peter Hansen Professor Susan Jarvis Sponsored by: Robot in Service of the Environment (RSE) 1.0 Introduction 2 2.0 Global Impact 4 2.1 Global Lionfish Populations 4 2.1.1 Lionfish Populations in the Western Atlantic 4 2.1.2 Lionfish Population in the Mediterranean 6 2.2 Invasive Impact in the Western Atlantic 6 2.2.1 Environmental Impact 7 2.2.2 Ecological Impact 7 2.2.3 Economical Impact 8 2.3 International Setting of the Caribbean 8 2.3.1 Historical Politics of the Caribbean 9 2.3.2 Caribbean Today 10 3.0 Caribbean Community Council 11 3.1 Community Council History 11 3.2 Community Composition 12 3.3 Community Structure and Operation 13 3.3.1 Community Organs 13 3.3.2 Community Bodies 14 3.3.3 Community Institutions 14 3.4 Community Impact 14 4.0 Requirements and Design Impacts 16 4.1 Engineering Goals 16 4.2 Operating Environment 16 4.3 Legal Considerations 17 4.4 International Project Communication 18 4.5 Acceptance Criteria & Approval 19 5.0 Public Concern and Response 21 5.1 Project Media Attention 21 5.2 Animal Protection Advocates 22 5.3 Concerns about Autonomous Technology 23 6.0 Comprehensive Project Approach 25 6.1 Globally-Minded Engineering 25 6.2 Thinking Beyond the Problem 25 6.3 Cultural Considerations 26 6.4 Power of Public Opinion 27 6.5 Collective Solution 28 7.0 Conclusion 30 8.0 References 32 1 1.0 Introduction The introduction of a species to new areas outside their native habitat often has extensive impacts on the existing ecosystem balance.